Harmonic oscillator (quantum): Difference between revisions
imported>Paul Wormer No edit summary |
imported>Paul Wormer No edit summary |
||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
The prototype of a '''harmonic oscillator''' is a mass ''m'' vibrating back and forth around an equilibrium position. In [[quantum mechanics]], the one-dimensional harmonic oscillator is one of the few systems that can be treated exactly. | The prototype of a one-dimensional '''[[harmonic oscillator (classical)|harmonic oscillator]]''' is a mass ''m'' vibrating back and forth on a line around an equilibrium position. In [[quantum mechanics]], the one-dimensional harmonic oscillator is one of the few systems that can be treated exactly, ''i.e.'', its [[Schrödinger equation]] can be solved analytically. | ||
Although the harmonic oscillator ''per se'' is not very important, a large number of systems are governed approximately by the harmonic oscillator equation. Whenever one studies the behavior of a physical system in the neighborhood of a stable equilibrium position, one arrives at equations which, in the limit of small oscillations, are those of a harmonic oscillator. Two well-known examples are the vibrations of the atoms in a diatomic molecule about their equilibrium position and the oscillations of atoms or ions of a crystalline lattice. Also the energy of [[electromagnetic wave]]s in a cavity can be looked upon as the energy of a large set of harmonic oscillators. | |||
The four lowest harmonic oscillator eigenfunctions | ==Lowest wave functions== | ||
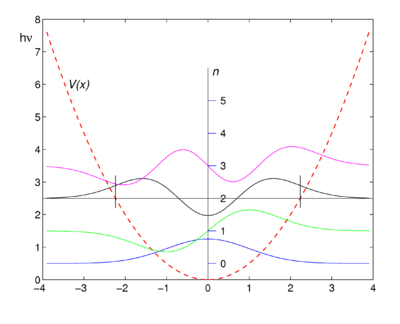
As stated above the Schrödinger equation of the one-dimensional quantum harmonic oscillator can be solved exactly, yielding analytic forms of the wave functions (eigenfunctions of the energy operator). The four lowest energy harmonic oscillator eigenfunctions are shown in the figure below. The functions are shifted upward such that their energy eigenvalues coincide with the asymptotic levels, the zero levels of the wave functions at ''x'' = ±∞. As an example of a zero level, the energy of the ''n'' = 2 function (black) is plotted, the horizontal line ''E''<sub>2</sub> = 5hν/2. | |||
According to quantum mechanics, the wave function squared of a point mass, |ψ(''x'')|², is the probability of finding the mass in the point ''x''. In the figure we see that the probability of finding the oscillating mass at points to the left or to the right of ''V''(''x'') is non-zero and we notice that for these points the potential energy ''V''(''x'') is larger than the energy eigenvalue. As an example two small vertical lines are plotted: to the left of the | According to quantum mechanics, the wave function squared of a point mass, |ψ(''x'')|², is the probability of finding the mass in the point ''x''. In the figure we see that the probability of finding the oscillating mass at points to the left (for negative ''x'') or to the right (for positive ''x'') of ''V''(''x'') is non-zero and we notice that for these points the potential energy ''V''(''x'') is larger than the energy eigenvalue. As an example two small vertical lines are plotted: to the left of the small line that lies at negative ''x'' the potential energy is larger than <font style="vertical-align: baseline;"><math>E_2 = 5h\nu/2 \equiv \tfrac{5}{2} \hbar\omega</math></font>, the energy of the ''n'' = 2 level. The same holds for the region to the right of the small black line at positive ''x''. Note that the ''n'' = 2 wave function (black) is not zero in these regions. | ||
[[Image:Oscillator.png|right|thumb|400px|First four harmonic oscillator functions ψ<sub>''n''</sub>. Function values are shifted upward by the corresponding energy values <math>(n+\tfrac{1}{2})h\nu.</math> The quadratic potential ''V''(''x'') is shown as reference. Small vertical (black) lines indicate boundaries of the classical forbidden regions of the ''n''=2 level. ]] | |||
In classical mechanics the potential energy ''V'' can never surpass the total energy ''E'', because ''V'' = ''E'' − ''T'' and the classical kinetic energy ''T'' is non-negative, so that ''V'' ≤ ''E''. This is why the region, where the energy eigenvalue of an eigenfunction is smaller than the potential energy, is called a ''classically forbidden region''. The fact that the probability of finding a mass in a classically forbidden is non-zero, is often expressed by stating that the point mass can ''tunnel'' into the potential wall. This tunnel effect is one of the more intriguing aspects of quantum mechanics. | |||
==Schrödinger equation== | ==Schrödinger equation== | ||
Revision as of 07:03, 29 April 2009
The prototype of a one-dimensional harmonic oscillator is a mass m vibrating back and forth on a line around an equilibrium position. In quantum mechanics, the one-dimensional harmonic oscillator is one of the few systems that can be treated exactly, i.e., its Schrödinger equation can be solved analytically.
Although the harmonic oscillator per se is not very important, a large number of systems are governed approximately by the harmonic oscillator equation. Whenever one studies the behavior of a physical system in the neighborhood of a stable equilibrium position, one arrives at equations which, in the limit of small oscillations, are those of a harmonic oscillator. Two well-known examples are the vibrations of the atoms in a diatomic molecule about their equilibrium position and the oscillations of atoms or ions of a crystalline lattice. Also the energy of electromagnetic waves in a cavity can be looked upon as the energy of a large set of harmonic oscillators.
Lowest wave functions
As stated above the Schrödinger equation of the one-dimensional quantum harmonic oscillator can be solved exactly, yielding analytic forms of the wave functions (eigenfunctions of the energy operator). The four lowest energy harmonic oscillator eigenfunctions are shown in the figure below. The functions are shifted upward such that their energy eigenvalues coincide with the asymptotic levels, the zero levels of the wave functions at x = ±∞. As an example of a zero level, the energy of the n = 2 function (black) is plotted, the horizontal line E2 = 5hν/2.
According to quantum mechanics, the wave function squared of a point mass, |ψ(x)|², is the probability of finding the mass in the point x. In the figure we see that the probability of finding the oscillating mass at points to the left (for negative x) or to the right (for positive x) of V(x) is non-zero and we notice that for these points the potential energy V(x) is larger than the energy eigenvalue. As an example two small vertical lines are plotted: to the left of the small line that lies at negative x the potential energy is larger than , the energy of the n = 2 level. The same holds for the region to the right of the small black line at positive x. Note that the n = 2 wave function (black) is not zero in these regions.
In classical mechanics the potential energy V can never surpass the total energy E, because V = E − T and the classical kinetic energy T is non-negative, so that V ≤ E. This is why the region, where the energy eigenvalue of an eigenfunction is smaller than the potential energy, is called a classically forbidden region. The fact that the probability of finding a mass in a classically forbidden is non-zero, is often expressed by stating that the point mass can tunnel into the potential wall. This tunnel effect is one of the more intriguing aspects of quantum mechanics.
Schrödinger equation
The time-independent Schrödinger equation of the harmonic oscillator has the form
The two terms between square brackets are the Hamiltonian (energy operator) of the system: the first term is the kinetic energy operator and the second the potential energy operator. The quantity is Planck's reduced constant, m is the mass of the oscillator, and k is Hooke's spring constant. See the classical harmonic oscillator for further explanation of m and k.
The solutions of the Schrödinger equation are characterized by a vibration quantum number n = 0,1,2, .. and are of the form
with
Here
The functions Hn(x) are Hermite polynomials; the first few are:
The graphs of the first four eigenfunctions are shown in the figure. Note that the functions of even n are even, that is, , while those of odd n are antisymmetric
Solution of the Schrödinger equation
We rewrite the Schrödinger equation in order to hide the physical constants m, k, and h. As a first step we define the angular frequency ω ≡ √k/m, the same formula as for the classical harmonic oscillator:
Secondly, we divide through by , a factor that has dimension energy,
Write
and the Schrödinger equation becomes
Note that all constants are hidden in the equation on the right.
A particular solution of this equation is
We verify this
so that
We conclude that ψ0(y) ≡ exp(-y2/2) is an eigenfunction
with eigenvalue
This encourages us to try to find a total solution ψ(y) by a function of the form
Use the Leibniz formula
and
then we see
Divide through by −ψ0(y)/2 and we find the equation for f
The differential equation of Hermite with polynomial solutions Hn(y) is
Due to the appearance of the integer coefficient 2n in the last term the solutions are polynomials. We see that if we put
that we have solved the Schrödinger equation for the harmonic oscillator. The unnormalized solutions are


![{\displaystyle \left[-{\frac {\hbar ^{2}}{2m}}{\frac {\mathrm {d} ^{2}}{\mathrm {d} x^{2}}}+{\frac {1}{2}}kx^{2}\right]\psi =E\psi }](https://wikimedia.org/api/rest_v1/media/math/render/svg/3d9f0668b35308e56ecebd650d35557a417587e9)







![{\displaystyle {\frac {1}{2}}\left[-{\frac {\hbar ^{2}}{m}}{\frac {\mathrm {d} ^{2}}{\mathrm {d} x^{2}}}+m\omega ^{2}x^{2}\right]\psi =E\psi .}](https://wikimedia.org/api/rest_v1/media/math/render/svg/fa364b4f4b0b31d74933d03ba31283324a200e95)

![{\displaystyle {\frac {1}{2}}\left[-{\frac {\hbar }{m\omega }}{\frac {\mathrm {d} ^{2}}{\mathrm {d} x^{2}}}+{\frac {m\omega }{\hbar }}x^{2}\right]\psi ={\frac {E}{\hbar \omega }}\psi .}](https://wikimedia.org/api/rest_v1/media/math/render/svg/2c52ece1d81ae4551f61d09b90fc3a758743f8ec)

![{\displaystyle {\frac {1}{2}}\left[-{\frac {1}{\beta ^{2}}}{\frac {\mathrm {d} ^{2}}{\mathrm {d} x^{2}}}+\beta ^{2}x^{2}\right]\psi ={\mathcal {E}}\psi \quad \Longrightarrow \quad {\frac {1}{2}}\left[-{\frac {\mathrm {d} ^{2}}{\mathrm {d} y^{2}}}+y^{2}\right]\psi ={\mathcal {E}}\psi .}](https://wikimedia.org/api/rest_v1/media/math/render/svg/2d1d15092a4e7bb074de00b75c4b9275b33c4e54)


![{\displaystyle {\frac {1}{2}}\left[-{\frac {\mathrm {d} ^{2}}{\mathrm {d} y^{2}}}+y^{2}\right]e^{-y^{2}/2}={\frac {1}{2}}\left[e^{-y^{2}/2}\left(1-y^{2}\right)+y^{2}e^{-y^{2}/2}\right]={\frac {1}{2}}e^{-y^{2}/2}.}](https://wikimedia.org/api/rest_v1/media/math/render/svg/93a7d91461e3cd6e73134967273d97d75c50c217)
![{\displaystyle {\frac {1}{2}}\left[-{\frac {\mathrm {d} ^{2}}{\mathrm {d} y^{2}}}+y^{2}\right]\psi _{0}={\frac {1}{2}}\psi _{0},}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f1aeb8aa73a43901e4177ddbfdcfb0c947936dff)



![{\displaystyle {\frac {1}{2}}\left[-{\frac {d^{2}\psi _{0}}{dy^{2}}}+y^{2}\psi _{0}\right]f={\frac {1}{2}}\psi _{0}f\quad {\hbox{and}}\quad {\frac {d\psi _{0}}{dy}}=-y\psi _{0}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/cf2e580c99c1105a568c3c229230f09a3d3960c9)
![{\displaystyle {\frac {1}{2}}\left[-{\frac {d^{2}(\psi _{0}f)}{dy^{2}}}+y^{2}\psi _{0}f\right]={\frac {1}{2}}\psi _{0}f+y\psi _{0}{\frac {df}{dy}}-{\frac {1}{2}}{\frac {d^{2}f}{dy^{2}}}\psi _{0}={\mathcal {E}}\psi _{0}f.}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5b28e76d23647ec69af861bee1b0a4c6d132f231)



