Condensate polishing: Difference between revisions
imported>Milton Beychok (→History: Copy edit) |
Pat Palmer (talk | contribs) m (Text replacement - "United States" to "United States of America") |
||
| Line 50: | Line 50: | ||
The first synthetic industrial ion exchange material was developed by two more Germans, F. Harm and A. Rümpler, in 1903.<ref name=Helfferich/> In 1905, yet another German, R. Gans, developed a commercial process for water softening water using [[sodium aluminosilicate]] which he called ''zeolites''.<ref name=DeSilva/> | The first synthetic industrial ion exchange material was developed by two more Germans, F. Harm and A. Rümpler, in 1903.<ref name=Helfferich/> In 1905, yet another German, R. Gans, developed a commercial process for water softening water using [[sodium aluminosilicate]] which he called ''zeolites''.<ref name=DeSilva/> | ||
A spectacular evolution began in 1935 with the discovery by two English chemists, B.A. Adams and E.l. Holmes, that crushed [[phonograph]] records exhibited ion exchange properties. Their work led them to synthesis of [[Organic chemistry|organic]] ion exchange resins with much better properties than any of the previous materials.<ref name=Britannica/><ref name=DeSilva/><ref name=Helfferich/> New synthetic resins were developed during [[World War II]] by the former [[I.G. Farbenindustrie]] in German. After the war, companies in the [[United States]] and in England also developed improved synthetic resins. Today, nearly all industrial and laboratory applications of ion exchange are based on synthetic resins. | A spectacular evolution began in 1935 with the discovery by two English chemists, B.A. Adams and E.l. Holmes, that crushed [[phonograph]] records exhibited ion exchange properties. Their work led them to synthesis of [[Organic chemistry|organic]] ion exchange resins with much better properties than any of the previous materials.<ref name=Britannica/><ref name=DeSilva/><ref name=Helfferich/> New synthetic resins were developed during [[World War II]] by the former [[I.G. Farbenindustrie]] in German. After the war, companies in the [[United States of America]] and in England also developed improved synthetic resins. Today, nearly all industrial and laboratory applications of ion exchange are based on synthetic resins. | ||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
Revision as of 10:52, 2 February 2023
Condensate polishing is an ion exchange process used to purify the steam condensate produced in high-pressure steam generation facilities[1][2][3][4] such as those in large thermal power plants.[5] Steam condensate is the water formed by condensing the exhaust steam from the steam-driven turbines in thermal power plants and which is recycled for reuse as the major part of the steam generation feedwater.
Condensate polishing is a unique application of ion exchange resins that removes suspended and dissolved impurities[6] from the condensate. It is essential for the very stringent quality required of high-pressure steam generation feedwater.
The ion exchange process mechanism
Ion exchange is a reversible chemical reaction wherein ions (atoms or molecules that have lost or gained an electron and thus acquired an electrical charge) present in a solution are exchanged for a similarly charged ion attached to an immobile solid particle of ion exchange material.[7][8]
By charging (i.e., treating) ion exchange material with an acid, usually sulfuric acid (H2SO4) or hydrochloric acid (HCl), positively charged hydrogen ions (H+) are attached to sites on the ion exchange material. Those H+ ions will attract and exchange places with other positively charged ions (called cations) present in a solution brought into contact with the positively charged H+ ions.
Similarly, by charging ion exchange material with a caustic such as sodium hydroxide (NaOH), negatively charged hydroxide ions (OH-) are attached to sites on the ion exchange material. Those sites will attract and exchange places with other negatively charged ions (called anions) present in a solution brought into contact with the negatively charged OH- ions.
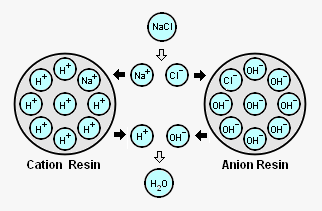
The adjacent diagram schematically depicts an acid treated bead of ion exchange resin with its attached H+ ions (referred to as cation resin) and a caustic treated bead of ion exchange resin with its attached OH- ions (referred to as an "anion resin").[9][10] As shown in the diagram, when those beads are contacted with sodium cations (Na+) and chlorine anions (Cl-) derived from dissolved sodium chloride (NaCl) in the steam condensate:
- The Na+ cations are attracted to the cation resin and exchange places with the H+ cations on the resin.
- The Cl- anions are attracted to the anion resin and exchange place with the OH- anions on the resin.
- The H+ cations removed from the cation resin and the OH- anions removed from the anion resin combine to form a molecule of water (H2O or HOH) that has no charge.
That illustrates how ion exchange material removes dissolved substances from steam condensate or any other solution. The process is sometimes referred to as demineralization (DM) or deionization (DI).
Once all of the charged ions attached on the cation and anion exchange materials have been replaced by ions removed from a solution, the ion exchange materials are referred to as spent. The spent materials can be regenerated (i.e., rejuvenated) by once again being charged with acid or caustic.
The ion exchange materials can be naturally occurring zeolites or synthetically produced polymeric resins. The synthetic resins are the most commonly used today because their characteristics can be tailored to specific applications.
Condensate polishing systems
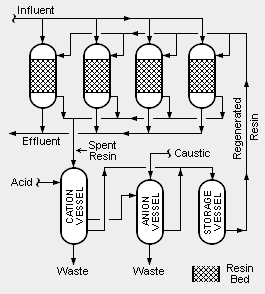
There are two different systems of condensate polishing. One uses pressure vessels containing mixed beds of cation and anion resins and the other uses pressure vessels containing powdered resins. Mixed bed systems (also referred to as deep bed systems), as illustrated in the adjacent schematic diagram, are the most commonly used systems.
In order to account for fluctuations in the amount of condensate to be treated and to provide continuous operation over long periods time, multiple vessels are used. For example, as shown in the adjacent diagram, there may be four onstream vessels containing beds of mixed resins. At any given time, three of the vessels are in onstream operation and one vessel is being regenerated. Each of the four vessels may be sized to handle 50 percent of the normal, average flow of condensate so that the three onstream vessels are capable of handling 150 percent of the average flow.
The incoming influent steam condensate is split into three parallel streams, with each stream flowing through one of the three onstream vessels. The treated outgoing effluent is recycled for use as feedwater to the steam generator(s).
In addition to removing dissolved impurities from the influent steam condensate, the beds of mixed ion exchange resins also act as filters to remove insoluble suspended impurities.
The system also includes three vessels used for regenerating spent resins. One uses acid to regenerate spent cation resin, another uses caustic to regenerate spent anion resin and the third is used for the intermediate storage of regenerated resin. The regeneration section produces wastewater (referred to as brine) that contains the reconstituted contaminants which were removed from the condensate feed water. Disposal of the waste brine can be a problem.
It is very important that resins being regenerated are first separated into cation resin and anion resin. There are many different methods for accomplishing that separation onsite. Alternatively, the separation may be done by offsite contractors.[3][4].
History
References to ion exchange have been attributed to the Old Testament scribes and later to Aristotle.[11] However, the first scientific descriptions have been credited to two English soil scientists, H.S. Thompson and J.T. Way who published their observations in 1850 about 'base' or cation exchange between calcium and ammonium ions on some types of soil.[12][13] In 1858, a German chemist, H. Eichhorn, proved that the ion exchange process in soil was a reversible one.[14][15][16]
Two other Germans, J. Lemberg in 1870 and later G. Wiegner in 1876, identified the soil material responsible for the observations of Thompson and Way as being clays, glauconites, zeolites and humic acids.[17] That led to the use of such materials in water softening processes and attempts to synthesize materials with similar properties. In 1896, natural zeolites were used for the first time in the sugar industry to replace sodium and potassium ions (Na+ and K+) by calcium and magnesium ions (Ca2+ and Mg2+) ions in sugar syrups.[15]
The first synthetic industrial ion exchange material was developed by two more Germans, F. Harm and A. Rümpler, in 1903.[17] In 1905, yet another German, R. Gans, developed a commercial process for water softening water using sodium aluminosilicate which he called zeolites.[14]
A spectacular evolution began in 1935 with the discovery by two English chemists, B.A. Adams and E.l. Holmes, that crushed phonograph records exhibited ion exchange properties. Their work led them to synthesis of organic ion exchange resins with much better properties than any of the previous materials.[13][14][17] New synthetic resins were developed during World War II by the former I.G. Farbenindustrie in German. After the war, companies in the United States of America and in England also developed improved synthetic resins. Today, nearly all industrial and laboratory applications of ion exchange are based on synthetic resins.
References
- ↑ Larry Drbal, Kayla Westra and Pat Boston (1996). Power Plant Engineering, 1st Edition. Springer. ISBN 0-412-06401-4.
- ↑ Brad Buecker (2000). Fundamentals of Steam Generation Chemistry, 1st Edition. Pennwell. ISBN 0-87814-750-0.
- ↑ 3.0 3.1 Condensate Polishing Guidelines Electric Power Research Institute (EPRI), 1996
- ↑ 4.0 4.1 Condensate Polishing State of Knowledge Assessment Electric Power Research Institute (EPRI), 2006
- ↑ Either nuclear or fuel-fired power plants
- ↑ The suspended impurities are usually referred to as Suspended solids and the dissolved impurities are often referred to as Total dissolved solids or TDS.
- ↑ Clive E. Harland (1994). Ion Exchange: Theory and Practice, 2nd Edition. Royal Society of Chemistry. ISBN 0-85186-484-8.
- ↑ Ion Exchange Basic Concepts
- ↑ Deionization Absorption and Ion Exchange Systems - Ion Exchange (DI)
- ↑ The Role of Ion Exchange Resins in Long-Term Spent Fuel Storage] Joseph Rideaux, 1998, Westinghouse Savannah River Company. A report of the U.S. Department of Energy. See Figure 3.
- ↑ Aristotle (ca. 330 B.C.), Works (reproduced and published by the Clarendon Press in 1927)
- ↑ In volume 11 of the Journal of the Royal Society of Agriculture of England (1850)
- ↑ 13.0 13.1 Ion-Exchange Reaction Encyclopedia Britannica
- ↑ 14.0 14.1 14.2 Essentials of Ion Exchange Francis J. DeSilva, 1999
- ↑ 15.0 15.1 The History of Chromatography Invited lecture by Anton Janßen, University of Applied Sciences, Münster, Germany, at the Commemorative Event for the 25th Chromatography Symposium – ChromForum – in Steinfurt, Germany on September 9, 2008
- ↑ H. Eichhorn, Poggendorff's Ann. Phys. Chem., 105 (1858) 126
- ↑ 17.0 17.1 17.2 Frederich G. Helfferich (1995). Ion Exchange. Dover Publications. ISBN 0-486-68784-8.