Dicrocoelium dendriticum: Difference between revisions
imported>Chris Day |
mNo edit summary |
||
| (61 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| Line 5: | Line 5: | ||
{{Taxobox | {{Taxobox | ||
| color = cyan | | color = cyan | ||
| name = '' | | name = ''Lancet fluke/Small liver fluke'' | ||
| image = | | image = Dicrocoelium_dendriticum3.jpg | ||
| regnum = Animalia | | regnum = Animalia | ||
| phylum = Platyhelminthes | | phylum = Platyhelminthes | ||
| Line 19: | Line 19: | ||
==Description and significance== | ==Description and significance== | ||
''Dicrocoelium dendriticum'' a small liver fluke, is a parasitic organism. That is, it benefits from its relationship to the host while contributing nothing to the survival of that host such that it in most cases may lower host fitness. Theory and mechanisms on parasite manipulation of host fitness is a current topic of much controversy (see | ''Dicrocoelium dendriticum'' a small liver fluke, is a parasitic organism. That is, it benefits from its relationship to the host while contributing nothing to the survival of that host such that it in most cases may lower host fitness. Theory and mechanisms on parasite manipulation of host fitness is a current topic of much controversy (see ‘Small Liver Fluke Manipulation of Host Behavior’ section below).Dicrocoeliosis is a globally present parasitic infection caused by ''Dicroceolium'', which infect the bile ducts and gall bladder of wild and domestic animals. Since it is not as pathogenic as other flukes very little is known about this parasite. Moreover, since it has two intermediate hosts Dicrocoeliosis is difficult to reproduce under experimental conditions. | ||
---- | |||
==Morphology == | |||
Dorso-ventrally flattned (lance shaped), adult lancet flukes are semi transparent ~8-14mm in length and ~2-3mm in width, oval shaped with the anterior slightly more narrow in shape compared to its posterior, both ends being slightly tapered <ref name=Desmond/>. At its tip the anterior contains an oral sucker. A hermaphrodite, the adult lancet fluke contains two lobed testes, in its anterior region, juxtaposed to its ovary, and the uterus lies below in its midsection. Vitellaria glands flank its reproductive organs, and are important in egg production. The digestive components (gut and bladder) lie in the posterior portion of its body<ref name=Krull>Krull, W. H., and C. R. Mapes. "Studies on the biology of Dicrocoelium dendriticum (Rudolphi, 1819) Looss, 1899 (Trematoda: Dicrocoeliidae), including its relation to the intermediate host, Cionella lubrica II. Collection of the snail, Cionella lubrica, and its maintenance in the laboratory." Cornell Vet 41 (1951): 433-44.</ref> | |||
---- | |||
==Parasitic Reservoirs: ''inside and outside the Definitive Host'' == | ==Parasitic Reservoirs: ''inside and outside the Definitive Host''[[Image:Zebrina Conchology A.jpg]][[Image:Intermediate hosts.jpg]]== | ||
''Dicrocoelium dendriticum’s '' habitat includes lowland or mountain pastures, of dry and alkaline consistency, providing appropriate conditions for its definitive and intermediate hosts ( | ''Dicrocoelium dendriticum’s '' habitat includes lowland or mountain pastures, of dry and alkaline consistency, providing appropriate conditions for its definitive and intermediate hosts (shown above).<ref name=Krull/> <ref name=Otranto/> The adult lancet fluke necessarily inhabits the liver of its definitive host, specifically the bile ducts and gall bladder of domesticated and wild ruminating animals (sheep, goat and cattle). Although, they can also be found in the liver of dogs, rabbits, horses, humans and some rodents <ref name=Otranto/>. Endogenous host and environmental conditions are critical for its survival, whereby each intermediate host must be favored in the collective biotope, making it difficult to model similar conditions in the laboratory. Since its continuance into the liver of its definitive host for sexual reproduction is dependent on prior passage through two other distinct host organisms, conditions must favor each part of this biological community. Once an adult, it uses its hermaphroditic body plan to propagate itself several orders of magnitude, but to get their the fluke must be ingested by specific intermediate hosts, which allow it to carry out the different stages of its life cycle <ref name=Otranto/>. | ||
---- | |||
==Life Cycle: ''A Definitive Host and Two Intermediate Hosts''== | ==Life Cycle: ''A Definitive Host and Two Intermediate Hosts''== | ||
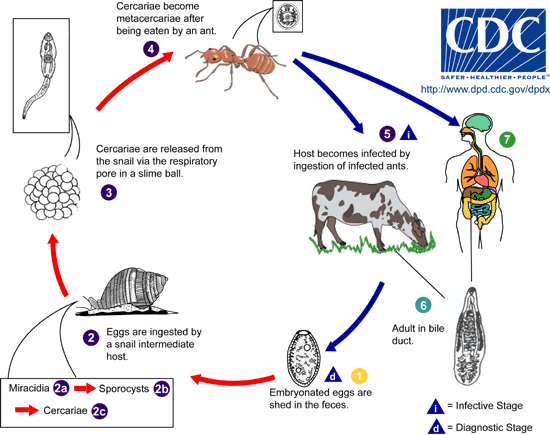
A trifecta of successful host:parasite adaptation, the small liver flukes’ life cycle and mode of transmission have been well described by the work of Krull and Mapes. Collectively their work showed that infection of the definitive host is initiated by ingestion of infected ants and cannot be bypassed by eating the slimeballs of infected snails, the first intermediate host | A trifecta of successful host:parasite adaptation, the small liver flukes’ life cycle and mode of transmission have been well described by the work of Krull and Mapes. Collectively their work showed that infection of the definitive host is initiated by ingestion of infected ants and cannot be bypassed by eating the slimeballs of infected snails, the first intermediate host.<ref name=Krull/><ref name=Otranto/><ref name=Desmond/>Adult flukes in the bile ducts, lay dark brown eggs each containing a miracidium, which are expelled with the bile into the intestine and later incorporated into the feces. These eggs are viable embryos, which are only hatched upon ingestion by the appropriate species of snail, Cionella lubrica in North America (There are many other possible host snail species depending on geographical location i.e. In France, Cochlicella acuta) <ref name=Otranto/>. The hatching mericidia penetrate the glandular intestinal epithelium and then undergo several rounds of asexual replication into daughter sporocysts <ref name=Krull/><ref name=Otranto/>. These daughter sporocysts mature into motile larva, called mature cercariae and travel to the snails’ respiratory chambers, a process that can take 5 months depending on season or age of the snail.<ref name=Krull/> <ref name=Otranto/> The cercariae contain glands which are speculated to be involved in the formation of slimeballs. Several ~500-5000 cercariae are collected in these slime accumulations but little is known about the mechanism of their formation.<ref name=Krull/><ref name=Otranto/> Slimeballs stick to nearby plants and debris, each snail usually produces one but can produce more.<ref name=Krull/> These slimeballs are ingested by a specific species of ant, in North America Formica fusca (Again, several species are capable of being intermediate host, differences being dependent on location). The larva then transform into metacercariae which grow in the abdomen of the ant. After a period of about a month <ref name=Desmond/>, at the expense of its own survival, a single metacercariae will become localized in the subesophageal ganglion, which results in changes in normal behavior of the ant, when temperatures are low <ref name=Krull/>. In this parasite adaptive state the infected ant climbs to the tips on blades of grass where they are exposed to grazing mammals such as sheep, goats and cows<ref name=Krull/> <ref name=Otranto/><ref name=Desmond/>. Finally, the metacercariae have arrived at their destination in the definitive host, where they are encysted in the duodenum <ref name=Otranto/>. The larva excyst and migrate to the bile ducts and then the gall bladder. In the bile ducts they develop into cross fertilizing and hermaphroditic adult flukes, capable of releasing new eggs into the environment, in the host excrement.<ref name=Krull/><ref name=Otranto/> <ref name=Desmond/> | ||
[[Image:Dicrocoelium LifeCycle.gif]] | |||
---- | |||
== | ==Epidemiology== | ||
''D. Dendriticum'' transmission depends on the presence of its intermediate hosts and the conditions that favor their survival. These conditions and thus the occurrence of ''D. dendriticum'' are dependent on; dry and alkaline soils <ref name=Otranto/>. Overall dicrocoeliosis is present sporadically in Europe, China, Japan, North Africa, South America, and some parts of North America, Australia and Africa. Although, it is endemic in parts of Spain, Brazil and a few Mediterranean countries.<ref name=Broglia/> These small liver flukes are found in dry lowland or mountain pastures, which provide sufficient conditions for the survival of snails and ants<ref name=Otranto/>. The prevalence of infection varies with season and the snail which is parasitized <ref name=Otranto/>. | |||
---- | |||
== | ==Pathology [[Image:D dendriticum egg wtmt JCG C.jpg]]== | ||
Disease occurrence in humans is rare, but infection by eating plants covered with infected ants (image of larval metacecariae egg, found in infected ant abdomen, is shown above or parasite manipulated ant on blade of grass shown below) or through ingestion of uncooked infected liver is possible. Although, in general livestock are at higher risk for infection. The small liver fluke has little identifiable clinical manifestations in infected hosts, compared other liver flukes i.e. ''Fasciola''. Even in heavily infected hosts they are asymptomatic, often suffering from anaemia, oedema or emaciation <ref name=Otranto/>. Also, the pathogenic effects of ''D. dendriticum'' can become confounded by simultaneous infections with other nematodes. Still some have been able to detect its effect on the liver <ref name=Otranto/>. In this case sheep liver was examined ''post mortum'' which appeared to have heavy scarring on its surface, hardening due to calcification and fibrosis, and displayed disruption of the bile ducts as a result of irritation caused by the presence of the small liver flukes <ref name=Otranto/>. | |||
---- | |||
==Small Liver Fluke Manipulation of Host Behavior== | |||
Out on the open plains, in the evening or just before sunrise a keen observer might see something quite remarkable. Several ants with their mandibles attached firmly to blades of grass. If their timing is right, they will later find themselves in a fatal journey culminating with their special hitchhikers (metacercariae) being transmitted successfully into the definitive hosts liver. If no livestock graze on these particular grasses then the ant will return to its normal duties in its colony<ref name=Krull/>. This manipulation of the second intermediate host is carried out when a mature cercaria in the ant abdomen matures into a | |||
metacercariae which is now motile and able to travel to the ant's subesophageal ganglion. As a result, the ant and the parasite (localized in the brain) are sacrificed<ref name=Krull/><ref name=Otranto/>. Clearly, there is a conflict of interest between host and parasite which only makes sense to the parasite. But, while the eggs of the parasite are passed on to the terminal host, the parasite which traveled to the ant brain had to sacrifice itself in the process. Thus this single motile parasite does not have the ability to pass on its genes. There is no model to explain this behavior but some have proposed that kin selection may be taking place and that the altruistic ant may share genes with other larva in the ant which will survive<ref name=Gandon/>. This behavior has gotten the attention of many biologist and those with scientific interest. Some have seen this as an example of how some adaptations while perfectly natural are clearly not beneficial to the intermediate host (the ant). This example of parasite manipulation of host behavior has been used by Biologist, Richard Dawkins and Philosopher, Dan Dennett, to explain how some adaptations while harmful, still propagate themselves quite readily from individual to individual. The latter conceptual example was used by these authors, in connection with how memes, units of cultural inheritance may transmit themselves, throughout the human population. | |||
[[Image:800px-Ant on leaf.jpg]] | |||
===Controversy: ''Cultural Diversity an Evolutionary Fluke?''=== | |||
= | The above mentioned example of parasite manipulation of host behavior has been cited by Richard Dawkins and Dan Dennett,In Dawkin's book ''The Extended Phenotype'' to explain how some adaptations while harmful, still propagate themselves quite readily from individual to individual <ref name=Dawkins/>. The concept of host manipulation was used, in connection with how memes, units of cultural inheritance (first mentioned in Dawkins' ''the Self Gene''), might transmit themselves amongst humans. Still this particular case of behavioral manipulation in ants is difficult to extrapolate to parasitic manipulation in humans. Especially, since this effect on ant behavior is only characterized in terms of physiological changes that take place in the ant once infected by the parasite and behavior and cognitive function in humans is highly complex. In any case, the field of Memetics is developing quickly and while still highly controversial it appears ideas and cultural practices, like religions, may also evolve by the process of natural selection (see Dan Dennetts TED talks [http://www.ted.com/index.php/talks/dan_dennett_on_dangerous_memes.html]and [http://www.ted.com/index.php/talks/dan_dennett_s_response_to_rick_warren.html]. | ||
---- | |||
==Current Research== | |||
==== Experimental ELISA for diagnosis of ovine dicrocoeliosis and application in a field survey A. Broglia et al. 2009==== | |||
==== | This group demonstrated that it was more efficient to serotype infected livestock versus waiting for a coprological sample. The latter being almost a month less sensitive post infection compared to the ELISA assay. The antigen, a small liver specific antigen, derived from an infected sheep's bile ducts, was previously isolated by another group and used for this study. | ||
==== Dicrocoeliosis of ruminants: a little known fluke disease D. Otranto et al. 2003==== | |||
This review mentions the lack of overall current study on ''D. dendriticum'' and review general information about the parasite. Briefly, it reviews the life cycle of D. Dendriticum which has two intermediate hosts, its epidemiology, a worldwide disease endemic in only some parts of Spain, South America a few Mediterranean countries. Also, the article mentions that the more practical forms of disease control would be better husbandry practices, pretreatment of free grazing animals likely to be infected and even introduce other animals to prey on the intermediate hosts. | |||
==== Hepatic marker enzymes, biochemical parameters and pathological effects in lambs experimentally infected with ''Dicroceolium dendriticum'' (Dignea) M.Y. Manga-Gonzalez et al. 2004 ==== | |||
This paper studied several qualitative and quantitative parameters post experimental parasitic infection. The authors quantified the levels of, key hepatic enzymes, number of worms post necropsy, and qualitatively examined histological slides of the infected lamb ''post mortum''. The authors found significant increases in hepatic enzyme activity ( ALT & AST), which the authors suspected was a pathogenic response to parasite invasion which produces host-toxic metabolites. | |||
---- | |||
==References== | ==References== | ||
<references/> | <references/> | ||
2. Otranto, Domenic Dicrocoeliosis of ruminants: a little known fluke disease Trends in Parasitology 2003 | |||
3. Desmond, James introduction to animal parisitology p. 214 Figure 14.14 | |||
4. Broglia, A. Experimental ELISA for diagnosis of ovine dicrocoeliosis and application in a field survey Parasitology Research 2009 | |||
5. Gandon, Sylvain Parasitic manipulation: a theoretical framework may help | |||
6. Dawkins, Richard The Extended Phenotype 1982, 1999 With a new afterword by Daniel Dennett | |||
==Links== | ==Links== | ||
1. http://www.fao.org/wairdocs/ILRI/x5492E/x5492e04.htm | 1. http://www.fao.org/wairdocs/ILRI/x5492E/x5492e04.htm | ||
2. http://www.weichtiere.at/Mollusks/Schnecken/parasitismus/dicrocoelium.html | |||
2. http://www.weichtiere.at/Mollusks/Schnecken/parasitismus/dicrocoelium.html[[Category:Suggestion Bot Tag]] | |||
Revision as of 06:00, 7 August 2024
| Lancet fluke/Small liver fluke | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | ||||||||||||||
| Scientific classification | ||||||||||||||
| ||||||||||||||
| Binomial name | ||||||||||||||
| Dicrocoelium dendriticum Rudolphi, 1819 |
Description and significance
Dicrocoelium dendriticum a small liver fluke, is a parasitic organism. That is, it benefits from its relationship to the host while contributing nothing to the survival of that host such that it in most cases may lower host fitness. Theory and mechanisms on parasite manipulation of host fitness is a current topic of much controversy (see ‘Small Liver Fluke Manipulation of Host Behavior’ section below).Dicrocoeliosis is a globally present parasitic infection caused by Dicroceolium, which infect the bile ducts and gall bladder of wild and domestic animals. Since it is not as pathogenic as other flukes very little is known about this parasite. Moreover, since it has two intermediate hosts Dicrocoeliosis is difficult to reproduce under experimental conditions.
Morphology
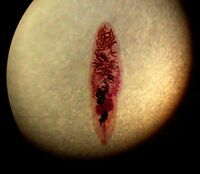
Dorso-ventrally flattned (lance shaped), adult lancet flukes are semi transparent ~8-14mm in length and ~2-3mm in width, oval shaped with the anterior slightly more narrow in shape compared to its posterior, both ends being slightly tapered [1]. At its tip the anterior contains an oral sucker. A hermaphrodite, the adult lancet fluke contains two lobed testes, in its anterior region, juxtaposed to its ovary, and the uterus lies below in its midsection. Vitellaria glands flank its reproductive organs, and are important in egg production. The digestive components (gut and bladder) lie in the posterior portion of its body[2]
Parasitic Reservoirs: inside and outside the Definitive Host

Dicrocoelium dendriticum’s habitat includes lowland or mountain pastures, of dry and alkaline consistency, providing appropriate conditions for its definitive and intermediate hosts (shown above).[2] [3] The adult lancet fluke necessarily inhabits the liver of its definitive host, specifically the bile ducts and gall bladder of domesticated and wild ruminating animals (sheep, goat and cattle). Although, they can also be found in the liver of dogs, rabbits, horses, humans and some rodents [3]. Endogenous host and environmental conditions are critical for its survival, whereby each intermediate host must be favored in the collective biotope, making it difficult to model similar conditions in the laboratory. Since its continuance into the liver of its definitive host for sexual reproduction is dependent on prior passage through two other distinct host organisms, conditions must favor each part of this biological community. Once an adult, it uses its hermaphroditic body plan to propagate itself several orders of magnitude, but to get their the fluke must be ingested by specific intermediate hosts, which allow it to carry out the different stages of its life cycle [3].
Life Cycle: A Definitive Host and Two Intermediate Hosts
A trifecta of successful host:parasite adaptation, the small liver flukes’ life cycle and mode of transmission have been well described by the work of Krull and Mapes. Collectively their work showed that infection of the definitive host is initiated by ingestion of infected ants and cannot be bypassed by eating the slimeballs of infected snails, the first intermediate host.[2][3][1]Adult flukes in the bile ducts, lay dark brown eggs each containing a miracidium, which are expelled with the bile into the intestine and later incorporated into the feces. These eggs are viable embryos, which are only hatched upon ingestion by the appropriate species of snail, Cionella lubrica in North America (There are many other possible host snail species depending on geographical location i.e. In France, Cochlicella acuta) [3]. The hatching mericidia penetrate the glandular intestinal epithelium and then undergo several rounds of asexual replication into daughter sporocysts [2][3]. These daughter sporocysts mature into motile larva, called mature cercariae and travel to the snails’ respiratory chambers, a process that can take 5 months depending on season or age of the snail.[2] [3] The cercariae contain glands which are speculated to be involved in the formation of slimeballs. Several ~500-5000 cercariae are collected in these slime accumulations but little is known about the mechanism of their formation.[2][3] Slimeballs stick to nearby plants and debris, each snail usually produces one but can produce more.[2] These slimeballs are ingested by a specific species of ant, in North America Formica fusca (Again, several species are capable of being intermediate host, differences being dependent on location). The larva then transform into metacercariae which grow in the abdomen of the ant. After a period of about a month [1], at the expense of its own survival, a single metacercariae will become localized in the subesophageal ganglion, which results in changes in normal behavior of the ant, when temperatures are low [2]. In this parasite adaptive state the infected ant climbs to the tips on blades of grass where they are exposed to grazing mammals such as sheep, goats and cows[2] [3][1]. Finally, the metacercariae have arrived at their destination in the definitive host, where they are encysted in the duodenum [3]. The larva excyst and migrate to the bile ducts and then the gall bladder. In the bile ducts they develop into cross fertilizing and hermaphroditic adult flukes, capable of releasing new eggs into the environment, in the host excrement.[2][3] [1]
Epidemiology
D. Dendriticum transmission depends on the presence of its intermediate hosts and the conditions that favor their survival. These conditions and thus the occurrence of D. dendriticum are dependent on; dry and alkaline soils [3]. Overall dicrocoeliosis is present sporadically in Europe, China, Japan, North Africa, South America, and some parts of North America, Australia and Africa. Although, it is endemic in parts of Spain, Brazil and a few Mediterranean countries.[4] These small liver flukes are found in dry lowland or mountain pastures, which provide sufficient conditions for the survival of snails and ants[3]. The prevalence of infection varies with season and the snail which is parasitized [3].
Pathology 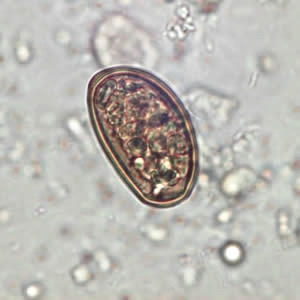
Disease occurrence in humans is rare, but infection by eating plants covered with infected ants (image of larval metacecariae egg, found in infected ant abdomen, is shown above or parasite manipulated ant on blade of grass shown below) or through ingestion of uncooked infected liver is possible. Although, in general livestock are at higher risk for infection. The small liver fluke has little identifiable clinical manifestations in infected hosts, compared other liver flukes i.e. Fasciola. Even in heavily infected hosts they are asymptomatic, often suffering from anaemia, oedema or emaciation [3]. Also, the pathogenic effects of D. dendriticum can become confounded by simultaneous infections with other nematodes. Still some have been able to detect its effect on the liver [3]. In this case sheep liver was examined post mortum which appeared to have heavy scarring on its surface, hardening due to calcification and fibrosis, and displayed disruption of the bile ducts as a result of irritation caused by the presence of the small liver flukes [3].
Small Liver Fluke Manipulation of Host Behavior
Out on the open plains, in the evening or just before sunrise a keen observer might see something quite remarkable. Several ants with their mandibles attached firmly to blades of grass. If their timing is right, they will later find themselves in a fatal journey culminating with their special hitchhikers (metacercariae) being transmitted successfully into the definitive hosts liver. If no livestock graze on these particular grasses then the ant will return to its normal duties in its colony[2]. This manipulation of the second intermediate host is carried out when a mature cercaria in the ant abdomen matures into a metacercariae which is now motile and able to travel to the ant's subesophageal ganglion. As a result, the ant and the parasite (localized in the brain) are sacrificed[2][3]. Clearly, there is a conflict of interest between host and parasite which only makes sense to the parasite. But, while the eggs of the parasite are passed on to the terminal host, the parasite which traveled to the ant brain had to sacrifice itself in the process. Thus this single motile parasite does not have the ability to pass on its genes. There is no model to explain this behavior but some have proposed that kin selection may be taking place and that the altruistic ant may share genes with other larva in the ant which will survive[5]. This behavior has gotten the attention of many biologist and those with scientific interest. Some have seen this as an example of how some adaptations while perfectly natural are clearly not beneficial to the intermediate host (the ant). This example of parasite manipulation of host behavior has been used by Biologist, Richard Dawkins and Philosopher, Dan Dennett, to explain how some adaptations while harmful, still propagate themselves quite readily from individual to individual. The latter conceptual example was used by these authors, in connection with how memes, units of cultural inheritance may transmit themselves, throughout the human population.
Controversy: Cultural Diversity an Evolutionary Fluke?
The above mentioned example of parasite manipulation of host behavior has been cited by Richard Dawkins and Dan Dennett,In Dawkin's book The Extended Phenotype to explain how some adaptations while harmful, still propagate themselves quite readily from individual to individual [6]. The concept of host manipulation was used, in connection with how memes, units of cultural inheritance (first mentioned in Dawkins' the Self Gene), might transmit themselves amongst humans. Still this particular case of behavioral manipulation in ants is difficult to extrapolate to parasitic manipulation in humans. Especially, since this effect on ant behavior is only characterized in terms of physiological changes that take place in the ant once infected by the parasite and behavior and cognitive function in humans is highly complex. In any case, the field of Memetics is developing quickly and while still highly controversial it appears ideas and cultural practices, like religions, may also evolve by the process of natural selection (see Dan Dennetts TED talks [1]and [2].
Current Research
Experimental ELISA for diagnosis of ovine dicrocoeliosis and application in a field survey A. Broglia et al. 2009
This group demonstrated that it was more efficient to serotype infected livestock versus waiting for a coprological sample. The latter being almost a month less sensitive post infection compared to the ELISA assay. The antigen, a small liver specific antigen, derived from an infected sheep's bile ducts, was previously isolated by another group and used for this study.
Dicrocoeliosis of ruminants: a little known fluke disease D. Otranto et al. 2003
This review mentions the lack of overall current study on D. dendriticum and review general information about the parasite. Briefly, it reviews the life cycle of D. Dendriticum which has two intermediate hosts, its epidemiology, a worldwide disease endemic in only some parts of Spain, South America a few Mediterranean countries. Also, the article mentions that the more practical forms of disease control would be better husbandry practices, pretreatment of free grazing animals likely to be infected and even introduce other animals to prey on the intermediate hosts.
Hepatic marker enzymes, biochemical parameters and pathological effects in lambs experimentally infected with Dicroceolium dendriticum (Dignea) M.Y. Manga-Gonzalez et al. 2004
This paper studied several qualitative and quantitative parameters post experimental parasitic infection. The authors quantified the levels of, key hepatic enzymes, number of worms post necropsy, and qualitatively examined histological slides of the infected lamb post mortum. The authors found significant increases in hepatic enzyme activity ( ALT & AST), which the authors suspected was a pathogenic response to parasite invasion which produces host-toxic metabolites.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Cite error: Invalid
<ref>tag; no text was provided for refs namedDesmond - ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 Krull, W. H., and C. R. Mapes. "Studies on the biology of Dicrocoelium dendriticum (Rudolphi, 1819) Looss, 1899 (Trematoda: Dicrocoeliidae), including its relation to the intermediate host, Cionella lubrica II. Collection of the snail, Cionella lubrica, and its maintenance in the laboratory." Cornell Vet 41 (1951): 433-44.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 Cite error: Invalid
<ref>tag; no text was provided for refs namedOtranto - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedBroglia - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedGandon - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedDawkins
2. Otranto, Domenic Dicrocoeliosis of ruminants: a little known fluke disease Trends in Parasitology 2003
3. Desmond, James introduction to animal parisitology p. 214 Figure 14.14
4. Broglia, A. Experimental ELISA for diagnosis of ovine dicrocoeliosis and application in a field survey Parasitology Research 2009
5. Gandon, Sylvain Parasitic manipulation: a theoretical framework may help
6. Dawkins, Richard The Extended Phenotype 1982, 1999 With a new afterword by Daniel Dennett
Links
1. http://www.fao.org/wairdocs/ILRI/x5492E/x5492e04.htm
2. http://www.weichtiere.at/Mollusks/Schnecken/parasitismus/dicrocoelium.html