Energostatic hypothesis: Difference between revisions
imported>Gareth Leng No edit summary |
mNo edit summary |
||
| (37 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{Image|Appetite.jpg|right|250px}} | |||
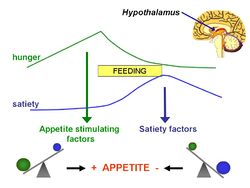
The '''energostatic hypothesis''' is based on the idea that, because the [[brain]] controls eating, it seems reasonable that [[hunger]] might be triggered by a decrease in the availability of energy for the brain itself.<ref>Booth DA (1972) Postabsorptively induced suppression of appetite and the energostatic control of feeding ''Physiol Behav'' 9:199–202 PMID 4654732</ref> | |||
''' | |||
==Introduction== | |||
The energostatic hypothesis considers how the availability of energy, the product of fuel metabolism, controls feeding behaviour. It had been accepted since the mid 1900's that glucose could mediate hunger levels, this was proposed to be through hepatic glucosensors in the liver that signalled directly to brain through the vagus nerve. It wasn't until Booth (1972) demonstrated that metabolites other than glucose also produced this effect that researchers began to question how this phenomenon came about. This is what initiated the belief that, through a common pathway, recent metabolism of fuels could control subsequent satiety levels and, ultimately, food intake. In this article we will discuss significant research findings that have shaped the hypothesis, the metabolic pathway linking the different fuels and the controversy surrounding the sensing of these metabolic signals. Importantly we will link these mechanisms to possible therapeutic targets in the management of obesity and indicate further research that needs undertaken before the significance of this hypothesis can be fully understood. | |||
==The dynamic between Glucose and Lipid systems== | |||
Extrapolating from evidence that glucose was able to control appetite, Booth believed these results could be related to the energy content of glucose and not specific to glucose as a fuel. He set out to test what became known as the 'energostatic hypothesis' by demonstrating that other energy-producing compounds could produce the same effects on feeding behaviour. As expected, glucose, [[glycerol]], [[alanine]] and [[lactate]] administration all reduced feeding by the expected energy yield of these fuels. This therefore indicated that these mice were not only able to monitor their metabolism, but that this was able to control subsequent food intake. This is thought to illustrate an intrinsic process to maintain short-term energy balance in mammals. It was proposed that [[metabolism]] must be sensed by receptors that are linked to hunger centres in the brain. This prompted the question of where these receptors were and what the common product of their metabolism pathways that was being sensed. | |||
[[Fatty acid oxidation]] was implicated in this process when it was discovered that Mercaptoacetate which inhibits fatty acid oxidation stimulated eating. It was also found that C75, which inhibits [[fatty acid synthase]], reduced food intake in mice by increasing fatty acid oxidation. This change is feeding behaviour was thought to mainly be in maintaining satiety following a meal as it is meal frequency and not meal size that is altered. This suggested that the link between the metabolism of different fuels was in the production of ATP. ATP or the AMP/ATP ratio as a measure of energy availability was thought to be able to control subsequent energy intake through feeding behaviour. Fat and glucose have synergistic responses in the control of feeding as inhibition of both of their metabolism pathways results in a greater increase in food intake then would be expected by the sum of their individual inhibition. This again suggests that they signal through a common mechanism. The hypothesis that the AMP/ATP ratio plays a significant role, consequently implicated AMPK, which detects changes in this ratio. It was also thought that the function of the liver in sensing and controlling the utilisation of glucose in addition to a role in fatty acid oxidation make it the likely location for the receptors involved in signalling. | |||
Because changes in the metabollic pathways alter both lipids and glucose the evidence strongly displays a clear link between the two substances. They are more powerful when their effects are observed together as opposed to seperaely. For example, it has been shown that inhibiting both pathways will produce a greater increase in feeding than individually showing that they most both play an important role. It also highlighted a prominent role for fatty acids and their oxidation towards food intake again suggesting the importance of the liver as it is very sensitive to any fatty acid inhibitor present (Friedman, 1995). | |||
==The role of AMPK== | |||
{{Image|AMPKon.jpg|right|250px|Image Caption}} | |||
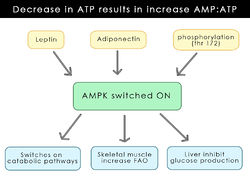
In recent years, a major development has occurred around the discovery of a protein kinase that is involved in the regulation of energy maintenance. Discovered in 1973, <ref>(Hardie and Carling, 1997)</ref>, the AMP-activated protein kinase (AMPK) regulates several key enzymes involved in fatty acid synthesis and steroid production. It is triggered by a decrease in the availability of ATP thereby increasing the AMP:ATP ratio. AMPK is activated to turn off certain ATP pathways that use up reserves in order to conserve energy and allows increased production of ATP in other systems, for example, the oxidation of fatty acids (Andersson ''et al.'' 2004). It is a complex process with many factors regulating its production (As displayed in figure 1-draw diagram to show its production). The initial thought was that it was released as a result of a stressor i.e., the lack of ATP. This process occurs at a cellular level but it seems there is now a more central role of AMPK involving the hypothalamic region and regulating appetite. There seems to be a correlation between AMPK and the hormones [[leptin]] and [[ghrelin]] which have opposite effects on energy uptake. While it is already known that these hormones decrease and increase food uptake respectively, it was also shown that AMPK is central to this process (Andersson ''et al.'' 2004). | |||
A recent study was carried out were concentrations of leptin and ghrelin were injected into rats to observe the effects of AMPK in the hypothalamus. AMPK was decreased when leptin was injected, while ghrelin caused an increase in production. The effects were observed approximately one hour after initial exposure to hormones and results were further quantified by measuring production of a downstream product of AMPK, ACC which showed similar significant effects as AMPK. The kinase was then further examined by altering its own production on energy uptake. A mimic of ATP, called ZMP, was synthesised and inserted into the PVN of the hypothalamus (a well-known site of energy uptake and expenditure) and into the third cerebral ventricle. As ZMP could mimic ATP it was able to stimulate production of AMPK. Results showed that it was indeed stimulated and food uptake was increased over an eight hour monitoring period showing that AMPK can have a direct influence over energy intake as well as showing a connection to other satiety hormones (Andersson ''et al.'' 2004). | |||
==Where is this mechanism triggered?== | |||
A lot of research has been conducted into the important of hepatic regulation into energy metabolism. Is this the main sensing organ or are higher centres more crucial in this process. | |||
It was initially believed that the liver controlled signals sent to the brain concerning energy availability by changes in the AMP/ATP ratio affecting vagal nerve afferents. In line with this view, glucose administered into the jugular vein did not inhibit feeding as well as its administration into the hepatic vein. This view is controversial for many reasons. The liver contains very few vagal nerve fibres thus, it was very unclear as to how such few fibres were able to sense these changes and how they actually resulted in a nerve response. Inconsistent findings were uncovered in the feeding response to liver fatty acid oxidation levels when interfered with using MA, which is known to inhibit fatty acid oxidation. Increased hepatic fatty acid oxidation reduced food intake but this behaviour failed to be reversed by MA. MA was also found to have the same inhibitory effects on food intake even when administered through the jugular vein, effectively bypassing the liver. These results cast huge doubts over whether the liver did play a role in the sensing of fatty acid oxidation that controlled feeding behaviour. | |||
Other researchers have proposed that cells of the proximal small intestine, the [[duodenum]], may be more appropriately placed to sense incoming metabolic load: ''...these enterocytes also take part in fatty acid oxidation and are thought to have a capacity increase FAO rate if required. This ability to alter fatty acid oxidation in response to increase demand placed on the system may actually determine a person’s sensitivity to obesity.'' This area of the small intestine also has vagal afferent fibres and the mucosal terminals are responsive to chemical stimuli, including ATP. However the role of enterocytes in the energostatic hypothesis has not been proven and the search for the ultimate sensor of energy availability through metabolism continues. | |||
For a metabolic event to influence eating behaviour, a sensory system must be present to detect metabolic changes and signal them to the brain where alterations to our feeding behaviour are made. | |||
The unique location of the liver and its role in managing glucose and fat fuels makes it an ideal candidate for assessing the consequences of ingestion and the sensation of satiety. Through infusion experiments, Tordoff and Friedman demonstrated that the administration of glucose into the hepatic portal vein suppressed food intake of freely-feeding rats more effectively than an infusion of glucose into the jugular vein. The amount of glucose entered was similar to the normal effects and loading of glucose after a normal meal. Increasing and decreasing the amounts of glucose inserted into the vein also produced a subsequent change in satiety leading to the idea that this mechanism is being regulated by the liver. This was also true when others metabolic substances such as fructose and glucagon were used. To test whether the liver is regulating the process a fructose analog (2,5-AM) was used as it can 'freeze' glucose metabolism and is taken up mainly by the liver. Infusions of this substance into the hepatic portal vein encouraged an increased feeding behaviour despite the doses given. Also, when the portal vein was removed there was a complete suppression of eating and an inhibtion of the neurons that usually respond to these signals showing that the liver is sensing the changed in energy uptake and relaying these messages to the CNS using sensory nerves within the hepatic portal vein (Friedmann, 1994). | |||
==Short/Long term benefits== | |||
We know that the energostatic hypothesis allows satiety for a period of time but are there any long-term benefits to this mechanism. | |||
It appears that the energostatic hypothesis can control short term feeding behaviours related to meal frequency and meal size but it does not seem to have a major impact over long term energy balance. Thus, the ability to override this satiety signal derived from fuel metabolism by sensory systems including olfaction and taste could diminish the significance of this hypothesis in controlling energy balance in real life feeding environment and behaviour. A suggested process of fuel partitioning may be relevant here. This considers the outcome of whether fuels are stored or oxidised in relation to alterations in ATP levels that could affect feeding behaviour. It is known that an increase in energy intake it usually paralleled with an increase in energy expenditure. This is thought to be due to fuels being taken away from the oxidative pathways, reducing the ATP levels and thus increasing the signal to eat. It has been seen that in the development of obesity that there is a change in this fuel partitioning before there is actually a change in the persons eating behaviour. In this case the increase in food intake is an appropriate stimulus from a reduction in the oxidation of fuels already taken into the body and stored. | |||
Some research has shown that other substances must be acting to control feeding as well as glucose. One factor that has been widely studied is insulin which was used alongside glucose to determine its effectiveness as a short-term regulator of appetite. One such study compared the use of a glucose load and a glucose-plus-insulin load of determining utilization of energy and feeding behaviour. The results suggested that many different nutrients are required in energy balance and not just the control of glucose is involved in the short-term feeding patterns. This study was one of the first to propose that the energostatic hypothesis was related to more than glucose loading and expenditure. | |||
==Current or Future Directions== | |||
The energostatic hypothesis suggests that we all monitor our metabolism in order to control energy balance, an intrinsic process ultimately developed to prevent obesity. The fact that obesity resistant mice are able to up regulate fatty acid oxidation may indicate a susceptibility factor in developing obesity and point to new ways in controlling obesity or even preventing it, if FAO can be pharmacologically manipulated appropriately. Modifying fuel partitioning could also be a focus of obesity management in researching ways to shift deposition from storage to oxidation pathways. | |||
==References== | ==References== | ||
<references/> | <references/>[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 11:00, 12 August 2024
The energostatic hypothesis is based on the idea that, because the brain controls eating, it seems reasonable that hunger might be triggered by a decrease in the availability of energy for the brain itself.[1]
Introduction
The energostatic hypothesis considers how the availability of energy, the product of fuel metabolism, controls feeding behaviour. It had been accepted since the mid 1900's that glucose could mediate hunger levels, this was proposed to be through hepatic glucosensors in the liver that signalled directly to brain through the vagus nerve. It wasn't until Booth (1972) demonstrated that metabolites other than glucose also produced this effect that researchers began to question how this phenomenon came about. This is what initiated the belief that, through a common pathway, recent metabolism of fuels could control subsequent satiety levels and, ultimately, food intake. In this article we will discuss significant research findings that have shaped the hypothesis, the metabolic pathway linking the different fuels and the controversy surrounding the sensing of these metabolic signals. Importantly we will link these mechanisms to possible therapeutic targets in the management of obesity and indicate further research that needs undertaken before the significance of this hypothesis can be fully understood.
The dynamic between Glucose and Lipid systems
Extrapolating from evidence that glucose was able to control appetite, Booth believed these results could be related to the energy content of glucose and not specific to glucose as a fuel. He set out to test what became known as the 'energostatic hypothesis' by demonstrating that other energy-producing compounds could produce the same effects on feeding behaviour. As expected, glucose, glycerol, alanine and lactate administration all reduced feeding by the expected energy yield of these fuels. This therefore indicated that these mice were not only able to monitor their metabolism, but that this was able to control subsequent food intake. This is thought to illustrate an intrinsic process to maintain short-term energy balance in mammals. It was proposed that metabolism must be sensed by receptors that are linked to hunger centres in the brain. This prompted the question of where these receptors were and what the common product of their metabolism pathways that was being sensed.
Fatty acid oxidation was implicated in this process when it was discovered that Mercaptoacetate which inhibits fatty acid oxidation stimulated eating. It was also found that C75, which inhibits fatty acid synthase, reduced food intake in mice by increasing fatty acid oxidation. This change is feeding behaviour was thought to mainly be in maintaining satiety following a meal as it is meal frequency and not meal size that is altered. This suggested that the link between the metabolism of different fuels was in the production of ATP. ATP or the AMP/ATP ratio as a measure of energy availability was thought to be able to control subsequent energy intake through feeding behaviour. Fat and glucose have synergistic responses in the control of feeding as inhibition of both of their metabolism pathways results in a greater increase in food intake then would be expected by the sum of their individual inhibition. This again suggests that they signal through a common mechanism. The hypothesis that the AMP/ATP ratio plays a significant role, consequently implicated AMPK, which detects changes in this ratio. It was also thought that the function of the liver in sensing and controlling the utilisation of glucose in addition to a role in fatty acid oxidation make it the likely location for the receptors involved in signalling.
Because changes in the metabollic pathways alter both lipids and glucose the evidence strongly displays a clear link between the two substances. They are more powerful when their effects are observed together as opposed to seperaely. For example, it has been shown that inhibiting both pathways will produce a greater increase in feeding than individually showing that they most both play an important role. It also highlighted a prominent role for fatty acids and their oxidation towards food intake again suggesting the importance of the liver as it is very sensitive to any fatty acid inhibitor present (Friedman, 1995).
The role of AMPK
In recent years, a major development has occurred around the discovery of a protein kinase that is involved in the regulation of energy maintenance. Discovered in 1973, [2], the AMP-activated protein kinase (AMPK) regulates several key enzymes involved in fatty acid synthesis and steroid production. It is triggered by a decrease in the availability of ATP thereby increasing the AMP:ATP ratio. AMPK is activated to turn off certain ATP pathways that use up reserves in order to conserve energy and allows increased production of ATP in other systems, for example, the oxidation of fatty acids (Andersson et al. 2004). It is a complex process with many factors regulating its production (As displayed in figure 1-draw diagram to show its production). The initial thought was that it was released as a result of a stressor i.e., the lack of ATP. This process occurs at a cellular level but it seems there is now a more central role of AMPK involving the hypothalamic region and regulating appetite. There seems to be a correlation between AMPK and the hormones leptin and ghrelin which have opposite effects on energy uptake. While it is already known that these hormones decrease and increase food uptake respectively, it was also shown that AMPK is central to this process (Andersson et al. 2004).
A recent study was carried out were concentrations of leptin and ghrelin were injected into rats to observe the effects of AMPK in the hypothalamus. AMPK was decreased when leptin was injected, while ghrelin caused an increase in production. The effects were observed approximately one hour after initial exposure to hormones and results were further quantified by measuring production of a downstream product of AMPK, ACC which showed similar significant effects as AMPK. The kinase was then further examined by altering its own production on energy uptake. A mimic of ATP, called ZMP, was synthesised and inserted into the PVN of the hypothalamus (a well-known site of energy uptake and expenditure) and into the third cerebral ventricle. As ZMP could mimic ATP it was able to stimulate production of AMPK. Results showed that it was indeed stimulated and food uptake was increased over an eight hour monitoring period showing that AMPK can have a direct influence over energy intake as well as showing a connection to other satiety hormones (Andersson et al. 2004).
Where is this mechanism triggered?
A lot of research has been conducted into the important of hepatic regulation into energy metabolism. Is this the main sensing organ or are higher centres more crucial in this process.
It was initially believed that the liver controlled signals sent to the brain concerning energy availability by changes in the AMP/ATP ratio affecting vagal nerve afferents. In line with this view, glucose administered into the jugular vein did not inhibit feeding as well as its administration into the hepatic vein. This view is controversial for many reasons. The liver contains very few vagal nerve fibres thus, it was very unclear as to how such few fibres were able to sense these changes and how they actually resulted in a nerve response. Inconsistent findings were uncovered in the feeding response to liver fatty acid oxidation levels when interfered with using MA, which is known to inhibit fatty acid oxidation. Increased hepatic fatty acid oxidation reduced food intake but this behaviour failed to be reversed by MA. MA was also found to have the same inhibitory effects on food intake even when administered through the jugular vein, effectively bypassing the liver. These results cast huge doubts over whether the liver did play a role in the sensing of fatty acid oxidation that controlled feeding behaviour.
Other researchers have proposed that cells of the proximal small intestine, the duodenum, may be more appropriately placed to sense incoming metabolic load: ...these enterocytes also take part in fatty acid oxidation and are thought to have a capacity increase FAO rate if required. This ability to alter fatty acid oxidation in response to increase demand placed on the system may actually determine a person’s sensitivity to obesity. This area of the small intestine also has vagal afferent fibres and the mucosal terminals are responsive to chemical stimuli, including ATP. However the role of enterocytes in the energostatic hypothesis has not been proven and the search for the ultimate sensor of energy availability through metabolism continues.
For a metabolic event to influence eating behaviour, a sensory system must be present to detect metabolic changes and signal them to the brain where alterations to our feeding behaviour are made.
The unique location of the liver and its role in managing glucose and fat fuels makes it an ideal candidate for assessing the consequences of ingestion and the sensation of satiety. Through infusion experiments, Tordoff and Friedman demonstrated that the administration of glucose into the hepatic portal vein suppressed food intake of freely-feeding rats more effectively than an infusion of glucose into the jugular vein. The amount of glucose entered was similar to the normal effects and loading of glucose after a normal meal. Increasing and decreasing the amounts of glucose inserted into the vein also produced a subsequent change in satiety leading to the idea that this mechanism is being regulated by the liver. This was also true when others metabolic substances such as fructose and glucagon were used. To test whether the liver is regulating the process a fructose analog (2,5-AM) was used as it can 'freeze' glucose metabolism and is taken up mainly by the liver. Infusions of this substance into the hepatic portal vein encouraged an increased feeding behaviour despite the doses given. Also, when the portal vein was removed there was a complete suppression of eating and an inhibtion of the neurons that usually respond to these signals showing that the liver is sensing the changed in energy uptake and relaying these messages to the CNS using sensory nerves within the hepatic portal vein (Friedmann, 1994).
Short/Long term benefits
We know that the energostatic hypothesis allows satiety for a period of time but are there any long-term benefits to this mechanism.
It appears that the energostatic hypothesis can control short term feeding behaviours related to meal frequency and meal size but it does not seem to have a major impact over long term energy balance. Thus, the ability to override this satiety signal derived from fuel metabolism by sensory systems including olfaction and taste could diminish the significance of this hypothesis in controlling energy balance in real life feeding environment and behaviour. A suggested process of fuel partitioning may be relevant here. This considers the outcome of whether fuels are stored or oxidised in relation to alterations in ATP levels that could affect feeding behaviour. It is known that an increase in energy intake it usually paralleled with an increase in energy expenditure. This is thought to be due to fuels being taken away from the oxidative pathways, reducing the ATP levels and thus increasing the signal to eat. It has been seen that in the development of obesity that there is a change in this fuel partitioning before there is actually a change in the persons eating behaviour. In this case the increase in food intake is an appropriate stimulus from a reduction in the oxidation of fuels already taken into the body and stored.
Some research has shown that other substances must be acting to control feeding as well as glucose. One factor that has been widely studied is insulin which was used alongside glucose to determine its effectiveness as a short-term regulator of appetite. One such study compared the use of a glucose load and a glucose-plus-insulin load of determining utilization of energy and feeding behaviour. The results suggested that many different nutrients are required in energy balance and not just the control of glucose is involved in the short-term feeding patterns. This study was one of the first to propose that the energostatic hypothesis was related to more than glucose loading and expenditure.
Current or Future Directions
The energostatic hypothesis suggests that we all monitor our metabolism in order to control energy balance, an intrinsic process ultimately developed to prevent obesity. The fact that obesity resistant mice are able to up regulate fatty acid oxidation may indicate a susceptibility factor in developing obesity and point to new ways in controlling obesity or even preventing it, if FAO can be pharmacologically manipulated appropriately. Modifying fuel partitioning could also be a focus of obesity management in researching ways to shift deposition from storage to oxidation pathways.
References
- ↑ Booth DA (1972) Postabsorptively induced suppression of appetite and the energostatic control of feeding Physiol Behav 9:199–202 PMID 4654732
- ↑ (Hardie and Carling, 1997)