Talk:Life/Draft/Building blocks: Difference between revisions
imported>Chris Day No edit summary |
Pat Palmer (talk | contribs) mNo edit summary |
||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 4: | Line 4: | ||
[http://discovermagazine.com/1993/oct/thetremblinggian285 The Trembling Giant] ''Discover Magazine'', October 1st | [http://discovermagazine.com/1993/oct/thetremblinggian285 The Trembling Giant] ''Discover Magazine'', October 1st | ||
:*From the article: "Yet even the majestic giant sequoia is not the record holder. That honor goes to a tree that my co-workers and I have studied for years: the quaking aspen, a common tree that dapples many mountains of North America. Unlike giant sequoias, each of which is a genetically separate individual, a group of thousands of aspens can actually be a single organism, sharing a root system and a unique set of genes. We therefore recently nominated one particular aspen individual growing just south of the Wasatch Mountains of Utah as the most massive living organism in the world. We nicknamed it Pando, a Latin word meaning "I spread". Made up of 47,000 tree trunks, each with an ordinary tree’s usual complement of leaves and branches, Pando covers 106 acres and, conservatively, weighs in excess of 13 million pounds…".</ref><ref>Zax D (2007) Champs. Smithsonian, Fall 2007. [http://www.nps.gov/archive/seki/shrm_pic.htm The General Sherman Tree] | :*From the article: "Yet even the majestic giant sequoia is not the record holder. That honor goes to a tree that my co-workers and I have studied for years: the quaking aspen, a common tree that dapples many mountains of North America. Unlike giant sequoias, each of which is a genetically separate individual, a group of thousands of aspens can actually be a single organism, sharing a root system and a unique set of genes. We therefore recently nominated one particular aspen individual growing just south of the Wasatch Mountains of Utah as the most massive living organism in the world. We nicknamed it Pando, a Latin word meaning "I spread". Made up of 47,000 tree trunks, each with an ordinary tree’s usual complement of leaves and branches, Pando covers 106 acres and, conservatively, weighs in excess of 13 million pounds…".</ref><ref>Zax D (2007) Champs. Smithsonian, Fall 2007. [http://www.nps.gov/archive/seki/shrm_pic.htm The General Sherman Tree] | ||
:*According to Smithsonian author David Zax, as of 2006 a California sequoia, the General Sherman Tree, ranks as the world's largest living organism (52,500 cubic feet, 2.7 million pounds). The world's oldest, a California bristlecone pine, Methuselah, >4,800 years old.</ref> It inspires wonder that particular collections of molecules, we humans, can generate words in the form of metaphors, in an attempt to explain the very activity of living that enables that feeling of wonder. Notwithstanding the molecular foundation of living things, | :*According to Smithsonian author David Zax, as of 2006 a California sequoia, the General Sherman Tree, ranks as the world's largest living organism (52,500 cubic feet, 2.7 million pounds). The world's oldest, a California bristlecone pine, Methuselah, >4,800 years old.</ref> It inspires wonder that particular collections of molecules, we humans, can generate words in the form of metaphors, in an attempt to explain the very activity of living that enables that feeling of wonder. Notwithstanding the molecular foundation of living things, the atoms and molecules, must first aggregate and organize as biological [[Cell|cells]] before anything living can emerge. | ||
Cells are considered the unit of life and living organisms can be either a single cell or a community of interacting cells. In living nucleated cells, organic molecules exist in heterogeneous pools of [[Materials science|colloidal]] ''[[Water|aqueous]]'' solutions bounded by [[lipid]]-[[protein]] membranes (e.g., nuclei, mitochondria, endoplasmic reticulum (see [[Cell (biology)|Cell]]). Each pool can have a different composition with distinct properties (e.g., transmembrane electrical potential difference; [[density]]; [[viscosity]]; [[Colligative properties|osmotic pressure]]; acidity; ionic strength) and different architectures. This heterogeneity provides the basis for the physiology that can cause electric fields, fluid shifts, energy transfers, and the transport of molecules into and out of the pools. | |||
Although organic molecules contain a variety of atomic elements (especially hydrogen, oxygen, nitrogen, phosphorus, and sulfur), they always have a predominant structure of carbon atoms, typically linked as carbon-to-carbon bonds in diverse topologies. | Although organic molecules contain a variety of atomic elements (especially hydrogen, oxygen, nitrogen, phosphorus, and sulfur), they always have a predominant structure of carbon atoms, typically linked as carbon-to-carbon bonds in diverse topologies. All cells share a common set of carbon-containing molecules - [[Organic chemistry|organic molecules]], dissolved or dispersed in [[Water|water]] as a common medium of housing and interaction — water comprises ~60-70% of the mature human organism. Those molecules include relatively small molecules, like [[amino acid|amino acids]], [[Nucleic acid metabolism|nucleotides]], [[monosaccharide]]s, and [[ester|esters]], and large ''macromolecules'' made up of sequences of smaller organic molecules. Organic macromolecules include [[protein]]s (sequences of amino acids), [[lipid]]s, [[Nucleic acid metabolism|nucleic acids]] (sequences of nucleotides), [[Macromolecular chemistry|polysaccharide]] (sequences of monosaccharides), and many other molecular genera. | ||
The 'stuff' of life, then, is carbon-to-carbon chains, studded with other atomic elements, arranged in aqueous lagoons containing a variety of organic and inorganic molecules, interacting in accord with physico-chemical principles. | |||
====Molecules==== | ====Molecules==== | ||
| Line 17: | Line 19: | ||
</font> '''''[http://mitpress.mit.edu/catalog/item/default.asp?ttype=2&tid=10907 --Jerome A. Feldman] | </font> '''''[http://mitpress.mit.edu/catalog/item/default.asp?ttype=2&tid=10907 --Jerome A. Feldman] | ||
|}{{-}} | |}{{-}} | ||
Why do [[carbon]] atoms play a central role in the chemistry of living things? Carbon has four electrons in its eight-electron-capacity outer shell, and it behaves ''as if'' it seeks four additional electrons to fill its outer shell to capacity (see accompanying figure and caption). Metaphorically speaking, it usually achieves its goal by forming "covalent bonds" with other atoms. The [[Physical chemistry|physical chemistry]] of carbon enables it to bond with many other elements with unfilled outer shells. Those include hydrogen, which can share one electron with carbon to fill its [hydrogen's] outer shell, allowing carbon to covalently bond to four hydrogen atoms, as in [[methane]] (CH<sub>4</sub>) [=natural gas]; oxygen, which can share two electrons with carbon to fill its [oxygen's] outer shell, allowing carbon to double-covalently bond with two oxygen atoms, as in [[carbon dioxide]] (CO<sub>2</sub>, or O=C=O; and nitrogen, which can share three electrons with carbon to fill its [nitrogen's] outer shell, allowing carbon to triple-covalently bond with one nitrogen atom, as in [[hydrocyanic acid]] (HCN). Most importantly, carbon can share electrons with itself, allowing the formation of C-C bonds, including double bonds (C=C) and triple bonds. The avidity for carbon to bond to itself allows carbon atoms to join into long chains, sometimes with C-C side chains, or even closed rings of C-C bonds, with or without side chains. Rings and chains and branches of linked carbons can combine into almost any imaginable shape. The particular covalent bonding capacity of carbon thus enables it to combine with hydrogen, oxygen, nitrogen, and itself in multi-varied ways that generate small carbon-based molecules such as [[sugar]]s, [[amino acid]]s and [[nucleotide]]s, which can join to become huge [[macromolecule]]s with remarkable stability. The sequences of the varied subunits of such macromolecules give them the informational content required for self-constructing the dynamic organization of cells and for constructing copies of themselves. | Why do [[carbon]] atoms play a central role in the chemistry of living things? Carbon has four electrons in its eight-electron-capacity outer shell, and it behaves ''as if'' it seeks four additional electrons to fill its outer shell to capacity (see accompanying figure and caption). Metaphorically speaking, it usually achieves its goal by forming "covalent bonds" with other atoms. The [[Physical chemistry|physical chemistry]] of carbon enables it to bond with many other elements with unfilled outer shells. Those include hydrogen, which can share one electron with carbon to fill its [hydrogen's] outer shell, allowing carbon to covalently bond to four hydrogen atoms, as in [[methane]] (CH<sub>4</sub>) [=natural gas]; oxygen, which can share two electrons with carbon to fill its [oxygen's] outer shell, allowing carbon to double-covalently bond with two oxygen atoms, as in [[carbon dioxide]] (CO<sub>2</sub>, or O=C=O; and nitrogen, which can share three electrons with carbon to fill its [nitrogen's] outer shell, allowing carbon to triple-covalently bond with one nitrogen atom, as in [[hydrocyanic acid]] (HCN). Most importantly, carbon can share electrons with itself, allowing the formation of C-C bonds, including double bonds (C=C) and triple bonds. The avidity for carbon to bond to itself allows carbon atoms to join into long chains, sometimes with C-C side chains, or even closed rings of C-C bonds, with or without side chains. Rings and chains and branches of linked carbons can combine into almost any imaginable shape. The particular covalent bonding capacity of carbon thus enables it to combine with hydrogen, oxygen, nitrogen, and itself in multi-varied ways that generate small carbon-based molecules such as [[sugar]]s, [[amino acid]]s and [[nucleotide]]s, which can join to become huge [[macromolecule]]s with remarkable stability. The sequences of the varied subunits of such macromolecules give them the informational content required for self-constructing the dynamic organization of cells and for constructing copies of themselves. | ||
| Line 26: | Line 27: | ||
The properties of carbon mean that organic macromolecules can contain huge 'banks' of information coded in their structure. Not only can each of the constituent molecules be huge, but several categories of chemicals, like [[Nucleic acid metabolism|nucleotides]] or [[amino acid]]s, that contain several different species, can be ordered so that the possible combinations are effectively limitless. All of these molecules are involved in the molecular-interaction networks of cells. | The properties of carbon mean that organic macromolecules can contain huge 'banks' of information coded in their structure. Not only can each of the constituent molecules be huge, but several categories of chemicals, like [[Nucleic acid metabolism|nucleotides]] or [[amino acid]]s, that contain several different species, can be ordered so that the possible combinations are effectively limitless. All of these molecules are involved in the molecular-interaction networks of cells. | ||
Amongst those networks of molecular interactions are those that enable cells to import and transform energy and energy-rich matter from the environment and that ultimately enable cells to grow, survive and reproduce. Matter needs energy to vitalize it. D'Arcy Thompson, a pioneering biologist in the early 20th century, considered talking about molecules (or matter generally) only provides convenience in that enables us to abbreviate the nomenclature and description of the energies and their forces that give the molecular assembly living status. | Amongst those networks of molecular interactions are those that enable cells to import and transform energy and energy-rich matter from the environment and that ultimately enable cells to grow, survive and reproduce. Matter needs energy to vitalize it. D'Arcy Thompson, a pioneering biologist in the early 20th century, considered talking about molecules (or matter generally) only provides convenience in that enables us to abbreviate the nomenclature and description of the energies and their forces that give the molecular assembly living status. | ||
Elsewhere in the universe, elements other than carbon and Earth-life's carbon-associated elements might give structure to living systems. Silicon, carbon's close columnar relative on the [[Periodic | Elsewhere in the universe, elements other than carbon and Earth-life's carbon-associated elements might give structure to living systems. Silicon, carbon's close columnar relative on the [[Periodic table of elements|periodic table]], also forms bond-chains with itself, forms covalent bonds with other elements, and supplies the basis for extraterrestrial living systems in fantasies by science fiction writers. Scientists conclude that silicon-silicon bonds do not stabilize under an Earth-like physico-chemical environment compatible with life as we know it.<ref name=bains04>Bains W. (2004) [http://dx.doi.org/10.1089/153110704323175124 Many Chemistries Could Be Used to Build Living Systems.] ''Astrobiology'' 4(2):137-167. | ||
:*In places in the universe where physical conditions might favor silicon-based macromolecules, silicon-based life might exist.</ref> Living systems, whether carbon-based or not, may not even require water to support the organization's chemistry.<ref>Ball P. (2005) [http://dx.doi.org/10.1038/4361084a Water and life: Seeking the solution.] ''Nature'' 436:1084-5]</ref> | :*In places in the universe where physical conditions might favor silicon-based macromolecules, silicon-based life might exist.</ref> Living systems, whether carbon-based or not, may not even require water to support the organization's chemistry.<ref>Ball P. (2005) [http://dx.doi.org/10.1038/4361084a Water and life: Seeking the solution.] ''Nature'' 436:1084-5]</ref> | ||
Latest revision as of 09:33, 26 September 2024
The fundamental units and processes of living things: the sine qua non
Building blocks
On Earth, everything living teems with vibrant molecules of myriad types and sizes, too small for the naked human eye to see, but numerous enough to come into view as a flea or a giant sequoia tree (up to 4.5 million pounds of molecules).[1][2] It inspires wonder that particular collections of molecules, we humans, can generate words in the form of metaphors, in an attempt to explain the very activity of living that enables that feeling of wonder. Notwithstanding the molecular foundation of living things, the atoms and molecules, must first aggregate and organize as biological cells before anything living can emerge.
Cells are considered the unit of life and living organisms can be either a single cell or a community of interacting cells. In living nucleated cells, organic molecules exist in heterogeneous pools of colloidal aqueous solutions bounded by lipid-protein membranes (e.g., nuclei, mitochondria, endoplasmic reticulum (see Cell). Each pool can have a different composition with distinct properties (e.g., transmembrane electrical potential difference; density; viscosity; osmotic pressure; acidity; ionic strength) and different architectures. This heterogeneity provides the basis for the physiology that can cause electric fields, fluid shifts, energy transfers, and the transport of molecules into and out of the pools.
Although organic molecules contain a variety of atomic elements (especially hydrogen, oxygen, nitrogen, phosphorus, and sulfur), they always have a predominant structure of carbon atoms, typically linked as carbon-to-carbon bonds in diverse topologies. All cells share a common set of carbon-containing molecules - organic molecules, dissolved or dispersed in water as a common medium of housing and interaction — water comprises ~60-70% of the mature human organism. Those molecules include relatively small molecules, like amino acids, nucleotides, monosaccharides, and esters, and large macromolecules made up of sequences of smaller organic molecules. Organic macromolecules include proteins (sequences of amino acids), lipids, nucleic acids (sequences of nucleotides), polysaccharide (sequences of monosaccharides), and many other molecular genera.
The 'stuff' of life, then, is carbon-to-carbon chains, studded with other atomic elements, arranged in aqueous lagoons containing a variety of organic and inorganic molecules, interacting in accord with physico-chemical principles.
Molecules
- See related topics: Chemistry, Biochemistry, and Organic Chemistry
|
From Molecule to Metaphor |
Why do carbon atoms play a central role in the chemistry of living things? Carbon has four electrons in its eight-electron-capacity outer shell, and it behaves as if it seeks four additional electrons to fill its outer shell to capacity (see accompanying figure and caption). Metaphorically speaking, it usually achieves its goal by forming "covalent bonds" with other atoms. The physical chemistry of carbon enables it to bond with many other elements with unfilled outer shells. Those include hydrogen, which can share one electron with carbon to fill its [hydrogen's] outer shell, allowing carbon to covalently bond to four hydrogen atoms, as in methane (CH4) [=natural gas]; oxygen, which can share two electrons with carbon to fill its [oxygen's] outer shell, allowing carbon to double-covalently bond with two oxygen atoms, as in carbon dioxide (CO2, or O=C=O; and nitrogen, which can share three electrons with carbon to fill its [nitrogen's] outer shell, allowing carbon to triple-covalently bond with one nitrogen atom, as in hydrocyanic acid (HCN). Most importantly, carbon can share electrons with itself, allowing the formation of C-C bonds, including double bonds (C=C) and triple bonds. The avidity for carbon to bond to itself allows carbon atoms to join into long chains, sometimes with C-C side chains, or even closed rings of C-C bonds, with or without side chains. Rings and chains and branches of linked carbons can combine into almost any imaginable shape. The particular covalent bonding capacity of carbon thus enables it to combine with hydrogen, oxygen, nitrogen, and itself in multi-varied ways that generate small carbon-based molecules such as sugars, amino acids and nucleotides, which can join to become huge macromolecules with remarkable stability. The sequences of the varied subunits of such macromolecules give them the informational content required for self-constructing the dynamic organization of cells and for constructing copies of themselves.
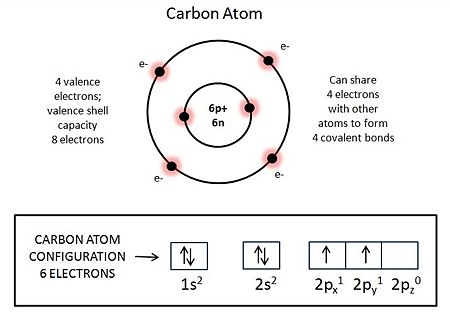
Atomic structure of the predominant isotope of a carbon atom: atomic number, Z=6; atomic mass = 12. Nucleus contains six protons (6p+) and six neutrons (6n). Electron configuration shown in rectangle. Outer shell (=valence shell) contains four electrons, has a capacity for eight electrons. The atom behaves as if it wants to fully fill its valence shell. With its valence shell fully occupied the atom achieves greatest stability as it has its least ability to react with other atoms. It usually achieves its valence shell octet of electrons by 'covalent' bonding, sharing electrons with other atoms, often with one or more other carbon atoms and one or more atoms of different elements also behaving as if they wanted to fill their own valence shells. See text.
The variety of carbon bonds vary in strength as well as in 3-D conformation. The simplest set of bonds that carbon can form is that of a tetrahedron, or pyramid, but the capacity of carbon for single, double and triple covalent bonding allows for many different geometries. Changing from one type of C-C bond to another type, as when a double bond is reduced to a single bond, will cause energy changes but without destroying the molecule. Such changes not only affect the molecule's energy state, but also affect the shape of the molecule and the particular side groups attached to it. One might say that the 'pulse of life' is represented at an atomic level.
The properties of carbon mean that organic macromolecules can contain huge 'banks' of information coded in their structure. Not only can each of the constituent molecules be huge, but several categories of chemicals, like nucleotides or amino acids, that contain several different species, can be ordered so that the possible combinations are effectively limitless. All of these molecules are involved in the molecular-interaction networks of cells.
Amongst those networks of molecular interactions are those that enable cells to import and transform energy and energy-rich matter from the environment and that ultimately enable cells to grow, survive and reproduce. Matter needs energy to vitalize it. D'Arcy Thompson, a pioneering biologist in the early 20th century, considered talking about molecules (or matter generally) only provides convenience in that enables us to abbreviate the nomenclature and description of the energies and their forces that give the molecular assembly living status.
Elsewhere in the universe, elements other than carbon and Earth-life's carbon-associated elements might give structure to living systems. Silicon, carbon's close columnar relative on the periodic table, also forms bond-chains with itself, forms covalent bonds with other elements, and supplies the basis for extraterrestrial living systems in fantasies by science fiction writers. Scientists conclude that silicon-silicon bonds do not stabilize under an Earth-like physico-chemical environment compatible with life as we know it.[3] Living systems, whether carbon-based or not, may not even require water to support the organization's chemistry.[4]
For the possibility of extraterrestrial life based on inorganic matter see novel proposal of physicists Tsytovich et al.[5][6] A mass of charged particles — like a swarm of bees — exhibiting features similar to Earth-type living systems
The possibility of non-molecular life, or life consisting of no matter at all (e.g., made up of energy fields), also interests science fiction writers. We science non-fiction writers consider energized molecules as the structural basis of living things on Earth.
Cells
- See Related Topics: Cell, Microbiology, Systems biology
|
"Omnis cellula e cellula" (Every cell out of a cell) |
In recognizing a living thing, biologists recognize it as a unity within an environment, yet apart from it — a compartment of a larger whole, structurally distinguishable though not functionally completely isolated from or closed to its surroundings. Every entity that biologists acknowledge as living — bacteria, trees, fish, chimpanzees — has a structurally compartmentalized building block, the biological cell. All cells extend themselves to (and include) an enclosing boundary that consists of a lipid-protein molecular membrane known as the cytoplasmic membrane, which structurally separates the interior of the cell from the external environment while allowing certain exchanges of energy and matter. The lipid molecules form the backbone of the cell membrane. [7]
Many organisms live as isolated cells, others as cooperative colonies of cells, and still others as complex multicellular systems that include diverse cell types, each specializing in different functions.[8] Nature has produced an enormous variety of cell types that span three vast ‘domains’ of living systems: Archaea, Bacteria, and Eukarya,[9] yet cells in all three domains have many features in common. In particular, as described above, they have a surrounding membrane, a physical boundary that separates them from their environment. (Yet that generally accepted commonality may oversimplify: see[10])
The detailed composition of cell membranes differ among cell types, with differing protein types and auxiliary lipid species, enabling specific kinds of functional exchanges with the surroundings. Pores, receptor molecules and protective walls are often features of the cell surface, in both unicellular and multicellular entities.[11]
Current evidence indicates that only pre-existing cells can ‘manufacture’ cells, so how did the first cell(s) arise? Examining what all cells have in common may provide insight to the origin of life. All extract energy from energy-rich molecules by simple oxidation reactions, and convert it into other, chemical forms of energy useful for cell function. The molecule ATP universally serves as the cell's main energy 'currency'. All cells inherit digitally stored information in the form of molecules of DNA, and with minor exceptions the DNA of all cells use the same universal genetic code to guide production of a myriad of distinct protein structures. Cells use those proteins to carry out diverse activities, including energy processing and conversion of carbon, nitrogen and phosphorous-containing materials into cellular structures. In the human genome, perhaps as few as 22,000 different protein-coding genes[12] lead to the production of many times more distinct protein structures that make up the variety and quantity of protein molecules needed for the structures and functions of a cell. Numerous molecular mechanisms account for that quantitative gene-to-protein amplification.[13]
Nature has produced a huge diversity of single-celled organisms and complex animals and plants. These can contain vast numbers of cells, each part of a specialized subpopulation (cell types) — in a mammal, the cells that make up bone differ in numerous structural and functional properties from those that make up muscle, and differ again from those that make up skin, for example. Humans contain approximately 200 different cell types as classified by microscopic anatomy.[8] In multicellular organisms, cells combine to make organs, the functional and structural components of the single larger organism.
What makes a single celled organism 'alive', and does the answer apply also when we call a large complex multicellular animal or plant 'alive'? What exactly do we mean by 'living'? We turn to those considerations next.
- ↑ Grant MC. (1993)
The Trembling Giant Discover Magazine, October 1st
- From the article: "Yet even the majestic giant sequoia is not the record holder. That honor goes to a tree that my co-workers and I have studied for years: the quaking aspen, a common tree that dapples many mountains of North America. Unlike giant sequoias, each of which is a genetically separate individual, a group of thousands of aspens can actually be a single organism, sharing a root system and a unique set of genes. We therefore recently nominated one particular aspen individual growing just south of the Wasatch Mountains of Utah as the most massive living organism in the world. We nicknamed it Pando, a Latin word meaning "I spread". Made up of 47,000 tree trunks, each with an ordinary tree’s usual complement of leaves and branches, Pando covers 106 acres and, conservatively, weighs in excess of 13 million pounds…".
- ↑ Zax D (2007) Champs. Smithsonian, Fall 2007. The General Sherman Tree
- According to Smithsonian author David Zax, as of 2006 a California sequoia, the General Sherman Tree, ranks as the world's largest living organism (52,500 cubic feet, 2.7 million pounds). The world's oldest, a California bristlecone pine, Methuselah, >4,800 years old.
- ↑ Bains W. (2004) Many Chemistries Could Be Used to Build Living Systems. Astrobiology 4(2):137-167.
- In places in the universe where physical conditions might favor silicon-based macromolecules, silicon-based life might exist.
- ↑ Ball P. (2005) Water and life: Seeking the solution. Nature 436:1084-5]
- ↑ Tsytovich VN et al. (2007) From plasma crystals and helical structures towards inorganic living matter. New J Phys 9:263]
- Note: From the Abstract: “Complex plasmas [a state of matter common in outer space consisting of a mass of charged particles] may naturally self-organize themselves into stable interacting helical structures that exhibit features normally attributed to organic living matter. The self-organization is based on non-trivial physical mechanisms of plasma interactions involving over-screening of plasma polarization. As a result, each helical string composed of solid microparticles is topologically and dynamically controlled by plasma fluxes leading to particle charging and over-screening, the latter providing attraction even among helical strings of the same charge sign. These interacting complex structures exhibit thermodynamic and evolutionary features thought to be peculiar only to living matter such as bifurcations that serve as `memory marks', self-duplication, metabolic rates in a thermodynamically open system, and non-Hamiltonian dynamics. We examine the salient features of this new complex `state of soft matter' in light of the autonomy, evolution, progenity and autopoiesis principles used to define life. It is concluded that complex self-organized plasma structures exhibit all the necessary properties to qualify them as candidates for inorganic living matter that may exist in space provided certain conditions allow them to evolve naturally."
- ↑ Editors (2007) 'It might be life, Jim...' Astrobiology Magazine Online, August 21, 2007.
- Astrobiology Magazine’s report for the general reader on the findings of Tsytovich et al. of self-organizing structures in plasma from inorganic dust. Remarkable images.
- ↑ Interestingly, among the three domains of living systems , the predominant lipid species differs. In most types of cells the predominant lipid species consist of molecules based on esters of glycerol combined with straight chain fatty acids, but in the Archaea domain it consists of ethers of glycerol combined with isoprene fatty components. That lack of membrane structural universality has implications for the origin of life.
- ↑ 8.0 8.1 Valentine JW et al. (1994) Morphological complexity increase in metazoans. Paleobiology 20:131-42
- ↑ Woese CR et al. (1990) Towards a natural system of organisms: proposal for the domains archaea, bacteria, and eucarya. Proc Natl Acad Sci USA 87:4576-9
- ↑ Baluska F et al. (2004) Eukaryotic cells and their cell bodies: cell theory revised. Ann Bot 94:9-32]
- Note: “…those who are aware of the most recent advances in plant cell biology…are convinced that Cell Theory, as it now stands, is absolutely incompatible with a cell-based organization of higher plants…and requires an update....Indeed, formulation of organismal theory of plant development, in which it is stated that it is not the cell but the whole multicellular organism that is the primary unit of plant life....has precipitated a crisis for Cell Theory as applied to plants. A consequence of the fact that the cytoplasms of plant cells are interconnected via plasmodesmata is that the individuality of the cell is given up in favour of an integrated and corporate cytoplasm that benefits the whole organism. This supracellular, or organismal, approach towards multicellularity seems to have allowed sessile plants to adapt to life on land and to evolve even within hostile environments.
- ↑ Note: Other boundaries of living systems include bark, shells, cell walls, skin, fur, and structures of the physical environment.
- ↑ How Many Genes Are in the Human Genome? at the Human Genome Project Information website hosted by the U.S. Department of Energy
- ↑ (a) The UniProtKB/Swiss-Prot Human Proteome Initiative; (b) Norregaard Jensen O (2004) Modification-specific proteomics: characterization of post-translational modifications by mass spectrometry. Curr Opin Chem Biol 8:33-41]