Rosuvastatin: Difference between revisions
imported>David E. Volk m (type II not class II) |
mNo edit summary |
||
| (8 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
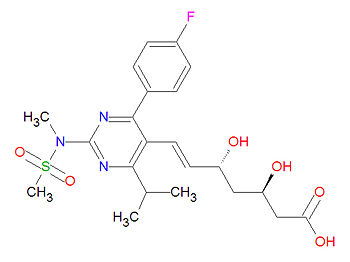
{{Image|Rosuvastatin structure.jpg|right|350px|Rosuvastatin, a type II statin.}} | |||
'''Rosuvastatin''', marketed as '''Crestor''', is a type II statin used to treat high cholesterol levels ([[hypercholesterolemia]]), prevent [[myocardial infarction|heart attacks]] and [[stroke]]s, and to diminish the formation of [[atherosclerosis|arterial plaque]]. It is a [[Hydroxymethylglutaryl-coenzyme A reductase inhibitor|HMG-CoA reductase inhibitor]] that decreases the conversion of HMG-CoA to [[mevalonate]], a key chemical precursor of [[cholesterol]]. It is related to other type II statins such as [[fluvastatin]] and [[atorvastatin]]. | '''Rosuvastatin''', marketed as '''Crestor''', is a type II [[statin]] used to treat high cholesterol levels ([[hypercholesterolemia]]), prevent [[myocardial infarction|heart attacks]] and [[stroke]]s, and to diminish the formation of [[atherosclerosis|arterial plaque]]. It is a [[Hydroxymethylglutaryl-coenzyme A reductase inhibitor|HMG-CoA reductase inhibitor]] that decreases the conversion of HMG-CoA to [[mevalonate]], a key chemical precursor of [[cholesterol]]. It is related to other type II statins such as [[fluvastatin]] and [[atorvastatin]]. | ||
Its official chemical IUPAC name is (E,3R,5R)-7-[4-(4-fluorophenyl)-2-(methyl-methylsulfonylamino)-6-propan-2-ylpyrimidin-5-yl]-3,5-dihydroxyhept-6-enoic acid and its chemical formula is C<sub>22</sub>H<sub>28</sub>FN<sub>3</sub>O<sub>6</sub>S. | Its official chemical IUPAC name is (E,3R,5R)-7-[4-(4-fluorophenyl)-2-(methyl-methylsulfonylamino)-6-propan-2-ylpyrimidin-5-yl]-3,5-dihydroxyhept-6-enoic acid and its chemical formula is C<sub>22</sub>H<sub>28</sub>FN<sub>3</sub>O<sub>6</sub>S. | ||
==History== | |||
Crestor brand of rosuvastatin was approved for Merck and Schering-Plough by the [[Food and Drug Administration]] in the [[United States of America]] with a [http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/NewDrugApplicationNDA/ New Drug Application] (NDA) in 2003.<ref>{{FDA-Drug_Details|021366}}</ref> A generic version of rosuvastatin calcium with a AB [[Food and Drug Administration/Catalogs/Therapeutic Equivalence Code|Therapeutic Equivalence Code]] was approved for Mylan Pharma with a [http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics/ Abbreviated New Drug Application] (ANDA) in 2010.<ref>{{FDA-Drug_Details|079161}}</ref> A generic version of rosuvastatin zinc with a AB [[Food and Drug Administration/Catalogs/Therapeutic Equivalence Code|Therapeutic Equivalence Code]] was approved for Watson Labs with a [http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics/ Abbreviated New Drug Application] (ANDA) in 2011.<ref>{{FDA-Drug_Details|202172}}</ref> | |||
==External links== | |||
{{CZMed|Rosuvastatin|6087}} | |||
==References== | |||
<references/>[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 11:01, 13 October 2024
Rosuvastatin, marketed as Crestor, is a type II statin used to treat high cholesterol levels (hypercholesterolemia), prevent heart attacks and strokes, and to diminish the formation of arterial plaque. It is a HMG-CoA reductase inhibitor that decreases the conversion of HMG-CoA to mevalonate, a key chemical precursor of cholesterol. It is related to other type II statins such as fluvastatin and atorvastatin.
Its official chemical IUPAC name is (E,3R,5R)-7-[4-(4-fluorophenyl)-2-(methyl-methylsulfonylamino)-6-propan-2-ylpyrimidin-5-yl]-3,5-dihydroxyhept-6-enoic acid and its chemical formula is C22H28FN3O6S.
History
Crestor brand of rosuvastatin was approved for Merck and Schering-Plough by the Food and Drug Administration in the United States of America with a New Drug Application (NDA) in 2003.[1] A generic version of rosuvastatin calcium with a AB Therapeutic Equivalence Code was approved for Mylan Pharma with a Abbreviated New Drug Application (ANDA) in 2010.[2] A generic version of rosuvastatin zinc with a AB Therapeutic Equivalence Code was approved for Watson Labs with a Abbreviated New Drug Application (ANDA) in 2011.[3]
External links
The most up-to-date information about Rosuvastatin and other drugs can be found at the following sites.
- Rosuvastatin - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Rosuvastatin - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Rosuvastatin - Detailed information from DrugBank.
References
- ↑ Anonymous. Drugs@FDA for FDA Application No. 021366. U S Food and Drug Administration
- ↑ Anonymous. Drugs@FDA for FDA Application No. 079161. U S Food and Drug Administration
- ↑ Anonymous. Drugs@FDA for FDA Application No. 202172. U S Food and Drug Administration