Steroid: Difference between revisions
imported>Gareth Leng No edit summary |
mNo edit summary |
||
| (29 intermediate revisions by 7 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{TOC | {{TOC|right}} | ||
'''Steroids''', or '''steroid hormones''', are powerful | '''Steroids''', or '''steroid hormones''', are [[hormone]]s with powerful effects, both beneficial and detrimental, when artificially supplemented into living systems. They are normally produced by three glands, the adrenal cortex, the testes, and the ovaries, but are also produced by the placenta during pregnancy, and some steroids ("neurosteroids") are produced within the brain. The five major classes of steroids are progestagens, [[glucocorticoids]], mineralcorticoids, androgens, and [[estrogen]]s. Steroids play an important role at all stages of life from the embryo until death. [[Corticosteroids]], or synthetic mimics such as [[prednisone]], are used to treat inflammation related illnesses like [[asthma]] or [[rheumatoid arthritis]], but they can have severe side effects. Athletes have often taken [[anabolic steroids]] to improve muscle growth and athletic performance. Glucocorticoids play a role in metabolism and inflammation, and estrogens have been linked to cancer. [[Testosterone]] and estrogen influence sexual traits (maleness/femaleness). All steroid hormones are naturally synthesized from [[cholesterol]] (through pregnenolone) under the control of the anterior pituitary gland, which produces [[andrenocorticotropic hormone]] (ACTH, or corticotropin), a polypeptide that stimulates the conversion of cholesterol to pregnenolone. The steroid hormones activate gene expression by binding to enhancer proteins, called [[steroid receptors]]. They also act through mechanisms not involving gene expression.<ref>Losel RM, Falkenstein ELIS, Feuring MART, Tillmann HC, Rossol-Haseroth KARI, Wehling MART. (2003) [http://physrev.physiology.org/cgi/content/abstract/83/3/965 Nongenomic Steroid Action: Controversies, Questions, and Answers.] ''Physiological Reviews'' 83(3):965-1016.</ref> Deficiency of the enzyme 21-hydroxylase is the most common steroid-related inborn metabolic disorder, but treatments are available for this condition, called congenital adrenal hyperplasia.<ref>[http://ghr.nlm.nih.gov/condition=21hydroxylasedeficiency What is 21-hydroxylase deficiency?] Genetics Home Reference, U.S. National Library of Medicine.</ref> | ||
==Classification and numbering == | ==Classification and numbering == | ||
Steroids can be classified into five major groups, based on their structures and functions:progestagens, glucocorticoids, mineralcorticoids, androgens and estrogens. The glucocorticoids and mineralcorticoids are referred to collectively as the corticosteroids, and the androgens and estrogens are referred to collectively as sex steroids. The | Steroids can be classified into five major groups, based on their structures and functions: ''progestagens, glucocorticoids, mineralcorticoids, androgens and estrogens''. The glucocorticoids and mineralcorticoids are referred to collectively as the 'corticosteroids', and the androgens and estrogens are referred to collectively as 'sex steroids' or 'gonadal steroids'. The progestagens are vital to the start of life, via pregnancy, while the glucocortioids and mineralocorticoids are vital to metabolism and electrolyte balance. The sex steroids provide the [[sexual dimorphism]] observed in many species. Because all steroids are derived from cholesterol, the numbering and nomenclature follow that of [[cholesterol]]. | ||
===Progestagens=== | ===Progestagens=== | ||
| Line 13: | Line 13: | ||
[[Image:Progesterone2.jpg|left|thumb|150px]] | [[Image:Progesterone2.jpg|left|thumb|150px]] | ||
Progesterone, a [[progestagen]] which prepares the lining of the uterus for implantation of an ovum, is biosynthesized from prognenolone by oxidation of the 3-hydroxy group into a 3-keto group and by the [[isomerization]] of the <math>\delta</math><sup>5</sup> double bond into a <math>\delta</math><sup>4</sup> double bond. This hormone is also essential to maintain pregnancy. Progesterone is the precursor chemical in the biosynthesis of corticoids and androgens. | |||
Progesterone, a [[progestagen]] which prepares the lining of the uterus for implantation of an ovum, is biosynthesized from prognenolone by oxidation of the 3-hydroxy group into a 3-keto group and by the [[isomerization]] of the <math>\delta</math><sup>5</sup> double bond into a <math>\delta</math><sup>4</sup> double bond. This hormone is also essential in mammals to maintain pregnancy. Progesterone is the precursor chemical in the biosynthesis of corticoids and androgens. | |||
==Biochemistry== | ==Biochemistry== | ||
| Line 21: | Line 23: | ||
===Corticosteroids=== | ===Corticosteroids=== | ||
{{Image|Cortisol corticosterone aldosterone stickfig DEVolk.jpg|right|450px|Structures of cortisol, the major glucocorticoid, corticosterone and aldosterone, the major mineralcorticoid.}} | |||
{{main|corticosteroid}} | {{main|corticosteroid}} | ||
====Biochemistry==== | ====Biochemistry==== | ||
| Line 28: | Line 30: | ||
====Medical use of glucocorticoids==== | ====Medical use of glucocorticoids==== | ||
{{main|glucocorticoid}} | {{main|glucocorticoid}} | ||
{{Image|Cortisone Prednisone stickfig DEVolk.jpg|right|250px|Prednisone is a cortisone mimic}} | |||
Although cortisone can be used to treat inflammation related diseases, like rheumatoid arthritis, it has severe side effects. The drug prednisone and other [[glucocorticoid]]s have been developed as a cortisone mimic and its use leads to milder side effect problems in the patient population. The only difference between the two compounds is the presence of an double bond between the C1 and C2 carbons of prednisone (note arrows in figure). Cortisone has a single C-C bond in this location. | Although cortisone can be used to treat inflammation related diseases, like rheumatoid arthritis, it has severe side effects. The drug prednisone and other [[glucocorticoid]]s have been developed as a cortisone mimic and its use leads to milder side effect problems in the patient population. The only difference between the two compounds is the presence of an double bond between the C1 and C2 carbons of prednisone (note arrows in figure). Cortisone has a single C-C bond in this location. | ||
===Sex steroids=== | ===Sex steroids=== | ||
[[Image:Androgen estrogen synthesis structures.jpg|right|thumb|400px|{{#ifexist:Template:Androgen estrogen synthesis structures.jpg/credit|{{Androgen estrogen synthesis structures.jpg/credit}}<br/>|}}]] | [[Image:Androgen estrogen synthesis structures.jpg|right|thumb|400px|{{#ifexist:Template:Androgen estrogen synthesis structures.jpg/credit|{{Androgen estrogen synthesis structures.jpg/credit}}<br/>|}}]] | ||
The term sex steroids refers collectively to the androgens and the estrogens, which both play a major role in defining sexual traits. Disorders affecting sex steroid levels can lead to the masculinization of women and the demasculinization of men. | The term sex steroids refers collectively to the androgens and the estrogens, which both play a major role in defining sexual traits. Disorders affecting sex steroid levels can lead to the masculinization of women and the demasculinization (feminization) of men. In [[androgen insensitivity syndrome]], a genetic mutation in the androgen receptor means that genetically male individuals (XY) do not respond normally to male hormones. In cases of complete insensitivity a genetic male will look like a female. | ||
====Biochemistry==== | ====Biochemistry==== | ||
Androgens and [[estrogen|estrogens]] have only nineteen or eighteen carbon atoms, respectively, compared to the larger progestagens, glucocorticoids, and mineralcorticoids, which all have twenty-one carbon atoms. The biosynthesis of both classes of sex steroids starts with the hydroxylation of progesterone at C-17 to produce 17<math>\alpha</math>-hydroxyprogesterone. Cleavage of the C20-C21 side chain and oxidation of the C-17 hydroxyl group into a ketone produces [[androstenedione]]. Reduction of the C-17 keto group of androstenedione yields [[testosterone]], one of the most abused [[anabolic steroid|anabolic steroids]]. | |||
The smallest class of steroids, the estrogens, are biosynthesized from the androgens by loss of the C-19 <math>\beta</math>-methyl group, reduction of the C-3 ketone to a hydroxyl group, and the formation of an aromatic ring A. [[Estradiol]] is the major estrogen. | Two major pathways metabolize testosterone into 17-keto steroids, primarily estradiol (E2) and dihydrotestosterone (DHT). DHT is further metabolized to 3<math>\alpha</math>- and 3<math>\beta</math>-androstanediol in reproductive tissues. | ||
The smallest class of steroids, the estrogens, are biosynthesized, by the enzyme [[aromatase]], from the androgens by loss of the C-19 <math>\beta</math>-methyl group, reduction of the C-3 ketone to a hydroxyl group, and the formation of an aromatic ring A. [[Estradiol]] is the major estrogen. | |||
====Medical use of androgens==== | ====Medical use of androgens==== | ||
Testosterone supplementation of elderly males with low serum testosterone levels has mixed results.<ref name="pmid18167405">{{cite journal |author=Emmelot-Vonk MH | In men, testosterone secretion declines with age, and this is associated with loss of muscle strength and sometimes loss of libido. Testosterone supplementation of elderly males with very low serum testosterone levels has mixed results.<ref name="pmid18167405">{{cite journal |author=Emmelot-Vonk MH ''et al'' |title=Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial |journal=JAMA |volume=299 |pages=39–52 |year=2008 |pmid=18167405 |doi=10.1001/jama.2007.51}}</ref> | ||
====Medical use of estrogens==== | ====Medical use of estrogens==== | ||
''For more information see [[estrogen]] and [[contraception (medical methods)]].'' | |||
At [[menopause]], circulating levels of estrogens decline markedly in women, and [[estrogen replacement therapy]] has been widely used to ameliorate the symptoms associated with the menopause. | |||
Presently, the only hormonal medications used for birth control are steroids aimed at manipulating the female [[ovulatory cycle]]. [[Progestin]] prevents ovulation by suppressing the secretion of [[luteinizing hormone]]. Progestins also thicken cervical mucus, thereby retarding the passage of sperm, and they make the endometrium unfavorable to implantation. [[Estrogen]] on the other hand prevents ovulation by suppressing the secretion of follicle-stimulating hormone (FSH), which is necessary to begin the ovulatory cycle. | |||
====Abuse of anabolic steroids==== | ====Abuse of anabolic steroids==== | ||
Anabolic steroids are steroids related to testosterone which encourage muscle development. They work by increasing the production of proteins and increase cell tissue, especially muscle mass. In addition, they decrease muscle catabolism by lowering the levels of [[cortisol]], a stress hormone. Anabolic steroids have other effects, including virilization, resulting in larger clitoris in women and penis in men, hair growth, acne, elevated blood pressure, liver and heart damage, and increases in LDL cholesterol and decreases in HDL cholesterol levels. The use of anabolic steroids is banned in many sports, and their use without valid prescriptions is illegal in many countries. | |||
===Mechanism of action=== | ===Mechanism of action=== | ||
Classically, steroids exert their effects by "genomic" actions - by altering gene expression in their target cells. Because of this mode of action, steroid hormones take hours to have an effect because messenger RNA and then proteins must be synthesized; this is in contrast with other fast acting hormones, such as [[epinephrine]], which | Classically, steroids exert their effects by "genomic" actions - by altering gene expression in their target cells. Because of this mode of action, steroid hormones take hours to have an effect because [[messenger RNA]] and then proteins must be synthesized; this is in contrast with other fast acting hormones, such as [[epinephrine]], in which their target cells have an immediate response. | ||
The genomic actions of steroids are mediated by specific receptors that are located in the [[cytosol]] of particular cells. Steroids enter cells freely since they are lipid soluble and so can pass through [[cell membrane]]s. All steroid hormone receptors have a hormone-binding region that binds the steroid tightly, with dissociation constants in the nanomolar range. The similarities in this region of the receptors suggests that they all had a common ancestor. The steroid receptor proteins are generally specific for each steroid class, binding tightly only to the one steroid, but weak binding can occur with some other steroids. | |||
In addition to these classic genomic actions, | This steroid:receptor complex then moves into the cell nucleus and binds to specific operator sites in the DNA. All steroid receptors have a DNA-binding region, often called the DNA-binding domain (or DBD), which is rich is the positively-charged amino acids arginine and lysine, and also contains many cysteines. The receptors also have activation factor domains that must interaction with additional cofactors before the steroid:receptor complex can enter the nucleus or initiate gene expression. Due to their complexity, hormone receptors are not well understood at this time and are the subject of intense research. Several steroid:receptor complex structures have recently been determined. | ||
In addition to these classic genomic actions, the steroids that give a more immediate response may have receptors on the cell membrane that initiate signal transduction. For example, in the brain, progesterone is metabolised into allopregnanolone which interacts with some classes of receptors for the inhibitory neurotransmitter [[GABA]]. | |||
==Diseases of steroid metabolism== | ==Diseases of steroid metabolism== | ||
===Adrenal insufficiency=== | ===Adrenal insufficiency=== | ||
{{main|Adrenal insufficiency}} | {{main|Adrenal insufficiency}} | ||
Adrenal insufficiency is a group of conditions in which the production of adrenal corticosteroids falls below the requirement of the body. Adrenal insufficiency can be caused by defects in the adrenal glands, the pituitary gland, or the hypothalamus. | |||
===Congenital Adrenal Hyperplasia=== | ===Congenital Adrenal Hyperplasia=== | ||
Congenital adrenal hyperplasia is a "group of inherited disorders of the adrenal glands, caused by enzyme defects in the synthesis of cortisol (hydrocortisone) and/or aldosterone leading to accumulation of precursors for androgens. Depending on the hormone imbalance, congenital adrenal hyperplasia can be classified as salt-wasting, hypertensive, virilizing, or feminizing. The most common defect is in steroid 21-hydroxylase. Other defects occur in steroid 11-beta-hydroxylase, steroid 17-alpha-hydroxylase, or 3-beta-hydroxysteroid dehydrogenase (3-hydroxysteroid dehydrogenases)".<ref name="title">{{cite web |url=http://www.nlm.nih.gov/cgi/mesh/2008/MB_cgi?term=Congenital+Adrenal+Hyperplasia |title=Adrenal Hyperplasia, Congenital |accessdate=2008-01-01 |author=National Library of Medicine |authorlink= |coauthors= |date= |format= |work= |publisher= |pages= |language= |archiveurl= |archivedate= |quote=}}</ref> | Congenital adrenal hyperplasia is a "group of inherited disorders of the adrenal glands, caused by enzyme defects in the synthesis of cortisol (hydrocortisone) and/or aldosterone leading to accumulation of precursors for androgens. Depending on the hormone imbalance, congenital adrenal hyperplasia can be classified as salt-wasting, hypertensive, virilizing, or feminizing. The most common defect is in steroid 21-hydroxylase. Other defects occur in steroid 11-beta-hydroxylase, steroid 17-alpha-hydroxylase, or 3-beta-hydroxysteroid dehydrogenase (3-hydroxysteroid dehydrogenases)".<ref name="title">{{cite web |url=http://www.nlm.nih.gov/cgi/mesh/2008/MB_cgi?term=Congenital+Adrenal+Hyperplasia |title=Adrenal Hyperplasia, Congenital |accessdate=2008-01-01 |author=National Library of Medicine |authorlink= |coauthors= |date= |format= |work= |publisher= |pages= |language= |archiveurl= |archivedate= |quote=}}</ref> | ||
| Line 65: | Line 79: | ||
;Manifestations | ;Manifestations | ||
Aldosterone is the main mineralcorticoid and is produced in the adrenal glands. It plays a critical role in | Aldosterone is the main mineralcorticoid and is produced in the adrenal glands. It plays a critical role in maintaining normal plasma concentrations of sodium; patients with 21-hydroxylase deficiency can suffer hypertension and dehydration, which can lead to shock or death. Increases in the levels of the androgens (testosterone, for example) resulting from the overproduction of progesterone and 17<math>\alpha</math>-hydroxy-progesterone lead to the [[virilization]] ((masculinization) of those affected. In girls, masculinization of the external genitalia is usually evident at birth. Boys appear normal at birth but sexual precocity soon follows. Accelerated growth and early bone development leads to a shortened stature. | ||
== | ==The Nobel Prize and steroid research== | ||
< | |||
Many scientists have won the Nobel Prize for work on these molecules. | |||
The 1927 Nobel Prize in chemistry was awarded to German chemist [[Heinrich Otto Wieland]] (1977-1957) "for his investigations of the constitution of the bile acids and related substances" "for the services rendered through his research into the constitution of the sterols and their connection with the vitamins" investigations he began in 1912 on the constitution of bile acids and related substances. He isolated three bile acids and discovered that they had similar structures and were structurally related to cholesterol. This work led to the recognition of the carbon framework of steroids and the study of oxidation processes in living cells. | |||
In 1928, the prize went to [[Adolf Otto Reinhold Windaus]] (1876-1959) | |||
"for the services rendered through his research into the constitution of the sterols and their connection with the vitamins"<ref>[http://nobelprize.org/nobel_prizes/chemistry/laureates/1928/windaus-bio.html Adolf Otto Reinhold Windaus]</ref> | |||
In 1939, [[Adolf Butenandt]] (1903-1995) was awarded the The Nobel Prize in Chemistry in 1939 for his work on sex hormones, jointly with [[Leopold Ruzicka]] for his work on polymethylenes and higher terpenes In <ref>[http://nobelprize.org/nobel_prizes/chemistry/laureates/1939/ruzicka-lecture.html Leopold Ruzicka]</ref>. Butenandt isolated [[oestrone]] in pure, crystalline form in 1929 and [[androsterone]] in 1931. From androsterone he (as well as Ruzicka, independently of each other) obtained [[testosterone]] in 1939. He isolated [[progesterone]] from the corpus luteum in 1934.<ref>[http://nobelprize.org/nobel_prizes/chemistry/laureates/1939/butenandt-bio.html Adolf Butenandt]</ref> | |||
In 1950, the Nobel Prize for Physiology or Medicine went jointly to [[Edward Kendall]] (1886-1972)<ref>[http://nobelprize.org/nobel_prizes/medicine/laureates/1950/kendall-bio.html Edward Kendall] </ref> , [[Tadeus Reichstein]] (1897-1976) <ref>[http://nobelprize.org/nobel_prizes/medicine/laureates/1950/reichstein-bio.html Tadeus Reichstein]</ref> and [[Philip Hench]] (1896-1965) <ref>[http://nobelprize.org/nobel_prizes/medicine/laureates/1950/hench-lecture.html Philip Hench]</ref> "for their discoveries relating to the hormones of the adrenal cortex, their structure and biological effects" | |||
<blockquote> | |||
:"Professor Edward Kendall. You and your collaborators have greatly contributed to the isolation and identification of the cortical hormones, and you have facilitated the artificial production of some of these substances. You have shown conclusively that their biological actions differ all according to their chemical structure. | |||
:"Professor Tadeus Reichstein. To you and your co-workers we owe the first isolation of four active hormones from the adrenal cortex, the first synthesis of one of them, the proof of the steroid nature of said hormones, and numerous details on the structure and properties of these important bodies. In this way the wearisome road to synthesis was smoothed, and new medicaments created. | |||
:"Doctor Philip Hench. Your brilliant investigations in respect of the beneficial effects of pregnancy and jaundice on rheumatoid arthritis have been the starting-point for the famous discovery during «the preceding year» that these diseases and some others are favourably influenced by hormones from the adrenal cortex." | |||
:(address by Professor G. Liljestrand) | |||
</blockquote> | |||
In 1969, [[Derek Barton]] (1918-1998) was awarded the Nobel Prize in Chemistry fore "contributions to the development of the concept of conformation and its application in chemistry". In 1950, in a short paper in ''Experientia'' entitled "The Conformation of the Steroid Nucleus", he had shown that organic molecules in general (and steroid molecules in particular) could be assigned a preferred conformation based upon results accumulated by chemical physicists, in particular by [[Odd Hassel]], with whom he shared the prize .<ref>[http://nobelprize.org/nobel_prizes/chemistry/laureates/1969/barton-bio.html Derek Barton]</ref> | |||
==See also== | ==See also== | ||
| Line 74: | Line 109: | ||
**[[Glucocorticoid]] | **[[Glucocorticoid]] | ||
[[Category: | ==References== | ||
{{reflist}}[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 11:00, 22 October 2024
Steroids, or steroid hormones, are hormones with powerful effects, both beneficial and detrimental, when artificially supplemented into living systems. They are normally produced by three glands, the adrenal cortex, the testes, and the ovaries, but are also produced by the placenta during pregnancy, and some steroids ("neurosteroids") are produced within the brain. The five major classes of steroids are progestagens, glucocorticoids, mineralcorticoids, androgens, and estrogens. Steroids play an important role at all stages of life from the embryo until death. Corticosteroids, or synthetic mimics such as prednisone, are used to treat inflammation related illnesses like asthma or rheumatoid arthritis, but they can have severe side effects. Athletes have often taken anabolic steroids to improve muscle growth and athletic performance. Glucocorticoids play a role in metabolism and inflammation, and estrogens have been linked to cancer. Testosterone and estrogen influence sexual traits (maleness/femaleness). All steroid hormones are naturally synthesized from cholesterol (through pregnenolone) under the control of the anterior pituitary gland, which produces andrenocorticotropic hormone (ACTH, or corticotropin), a polypeptide that stimulates the conversion of cholesterol to pregnenolone. The steroid hormones activate gene expression by binding to enhancer proteins, called steroid receptors. They also act through mechanisms not involving gene expression.[1] Deficiency of the enzyme 21-hydroxylase is the most common steroid-related inborn metabolic disorder, but treatments are available for this condition, called congenital adrenal hyperplasia.[2]
Classification and numbering
Steroids can be classified into five major groups, based on their structures and functions: progestagens, glucocorticoids, mineralcorticoids, androgens and estrogens. The glucocorticoids and mineralcorticoids are referred to collectively as the 'corticosteroids', and the androgens and estrogens are referred to collectively as 'sex steroids' or 'gonadal steroids'. The progestagens are vital to the start of life, via pregnancy, while the glucocortioids and mineralocorticoids are vital to metabolism and electrolyte balance. The sex steroids provide the sexual dimorphism observed in many species. Because all steroids are derived from cholesterol, the numbering and nomenclature follow that of cholesterol.
Progestagens
Pregnenolone is the first steroid derived from cholesterol. It is synthesized through an intermediate, 20,22-dihydroxy-cholesterol, which is subsequently oxidized at C-20 to form a ketone with cleavage of carbons 22-27.
Progesterone, a progestagen which prepares the lining of the uterus for implantation of an ovum, is biosynthesized from prognenolone by oxidation of the 3-hydroxy group into a 3-keto group and by the isomerization of the 5 double bond into a 4 double bond. This hormone is also essential in mammals to maintain pregnancy. Progesterone is the precursor chemical in the biosynthesis of corticoids and androgens.
Biochemistry
Synthesis
Cholesterol is the precursor from which all steroid hormones are synthesized. All steroid hormones have 21 or fewer carbons, although their precursor cholesterol has 27 carbon atoms. Because of this, all steroid numbering and nomenclature follow that of cholesterol. Some cholesterol derivatives have a proton added to the C-5 carbon. If the H-5 proton is -oriented (points down) then rings A and B are fused in a trans conformation, but in the orientation, the rings are fused in a cis conformation. All steroids with an H5 are in the 5 orientation, while bile salts derived from cholesterol have the opposite 5 orientation.
Corticosteroids
Biochemistry
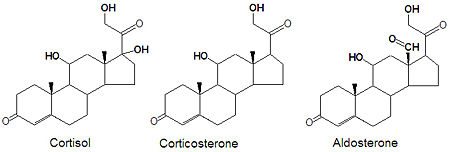
Glucocorticoids and mineralocorticoids (or mineralcorticoids) are naturally synthesized by the enzymatic oxidation of progesterone. Cortisol, the major glucocorticoid, is produced when progesterone is hydroxylated at three positions, C-11, C-17 and C21. The oxidation of the C-17 carbon must occur before the hydroxylation at C-21 to synthesize cortisol, otherwise corticosterone is formed. Aldosterone, the major mineralcorticoid, is synthesized from corticosterone by oxidation of the C-18 methyl group to form an aldehyde.
Medical use of glucocorticoids
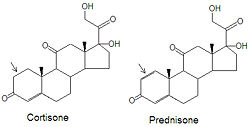
Although cortisone can be used to treat inflammation related diseases, like rheumatoid arthritis, it has severe side effects. The drug prednisone and other glucocorticoids have been developed as a cortisone mimic and its use leads to milder side effect problems in the patient population. The only difference between the two compounds is the presence of an double bond between the C1 and C2 carbons of prednisone (note arrows in figure). Cortisone has a single C-C bond in this location.
Sex steroids
The term sex steroids refers collectively to the androgens and the estrogens, which both play a major role in defining sexual traits. Disorders affecting sex steroid levels can lead to the masculinization of women and the demasculinization (feminization) of men. In androgen insensitivity syndrome, a genetic mutation in the androgen receptor means that genetically male individuals (XY) do not respond normally to male hormones. In cases of complete insensitivity a genetic male will look like a female.
Biochemistry
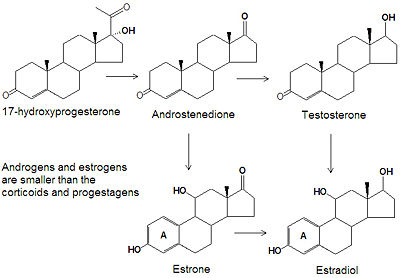
Androgens and estrogens have only nineteen or eighteen carbon atoms, respectively, compared to the larger progestagens, glucocorticoids, and mineralcorticoids, which all have twenty-one carbon atoms. The biosynthesis of both classes of sex steroids starts with the hydroxylation of progesterone at C-17 to produce 17-hydroxyprogesterone. Cleavage of the C20-C21 side chain and oxidation of the C-17 hydroxyl group into a ketone produces androstenedione. Reduction of the C-17 keto group of androstenedione yields testosterone, one of the most abused anabolic steroids.
Two major pathways metabolize testosterone into 17-keto steroids, primarily estradiol (E2) and dihydrotestosterone (DHT). DHT is further metabolized to 3- and 3-androstanediol in reproductive tissues.
The smallest class of steroids, the estrogens, are biosynthesized, by the enzyme aromatase, from the androgens by loss of the C-19 -methyl group, reduction of the C-3 ketone to a hydroxyl group, and the formation of an aromatic ring A. Estradiol is the major estrogen.
Medical use of androgens
In men, testosterone secretion declines with age, and this is associated with loss of muscle strength and sometimes loss of libido. Testosterone supplementation of elderly males with very low serum testosterone levels has mixed results.[3]
Medical use of estrogens
For more information see estrogen and contraception (medical methods).
At menopause, circulating levels of estrogens decline markedly in women, and estrogen replacement therapy has been widely used to ameliorate the symptoms associated with the menopause.
Presently, the only hormonal medications used for birth control are steroids aimed at manipulating the female ovulatory cycle. Progestin prevents ovulation by suppressing the secretion of luteinizing hormone. Progestins also thicken cervical mucus, thereby retarding the passage of sperm, and they make the endometrium unfavorable to implantation. Estrogen on the other hand prevents ovulation by suppressing the secretion of follicle-stimulating hormone (FSH), which is necessary to begin the ovulatory cycle.
Abuse of anabolic steroids
Anabolic steroids are steroids related to testosterone which encourage muscle development. They work by increasing the production of proteins and increase cell tissue, especially muscle mass. In addition, they decrease muscle catabolism by lowering the levels of cortisol, a stress hormone. Anabolic steroids have other effects, including virilization, resulting in larger clitoris in women and penis in men, hair growth, acne, elevated blood pressure, liver and heart damage, and increases in LDL cholesterol and decreases in HDL cholesterol levels. The use of anabolic steroids is banned in many sports, and their use without valid prescriptions is illegal in many countries.
Mechanism of action
Classically, steroids exert their effects by "genomic" actions - by altering gene expression in their target cells. Because of this mode of action, steroid hormones take hours to have an effect because messenger RNA and then proteins must be synthesized; this is in contrast with other fast acting hormones, such as epinephrine, in which their target cells have an immediate response.
The genomic actions of steroids are mediated by specific receptors that are located in the cytosol of particular cells. Steroids enter cells freely since they are lipid soluble and so can pass through cell membranes. All steroid hormone receptors have a hormone-binding region that binds the steroid tightly, with dissociation constants in the nanomolar range. The similarities in this region of the receptors suggests that they all had a common ancestor. The steroid receptor proteins are generally specific for each steroid class, binding tightly only to the one steroid, but weak binding can occur with some other steroids.
This steroid:receptor complex then moves into the cell nucleus and binds to specific operator sites in the DNA. All steroid receptors have a DNA-binding region, often called the DNA-binding domain (or DBD), which is rich is the positively-charged amino acids arginine and lysine, and also contains many cysteines. The receptors also have activation factor domains that must interaction with additional cofactors before the steroid:receptor complex can enter the nucleus or initiate gene expression. Due to their complexity, hormone receptors are not well understood at this time and are the subject of intense research. Several steroid:receptor complex structures have recently been determined.
In addition to these classic genomic actions, the steroids that give a more immediate response may have receptors on the cell membrane that initiate signal transduction. For example, in the brain, progesterone is metabolised into allopregnanolone which interacts with some classes of receptors for the inhibitory neurotransmitter GABA.
Diseases of steroid metabolism
Adrenal insufficiency
Adrenal insufficiency is a group of conditions in which the production of adrenal corticosteroids falls below the requirement of the body. Adrenal insufficiency can be caused by defects in the adrenal glands, the pituitary gland, or the hypothalamus.
Congenital Adrenal Hyperplasia
Congenital adrenal hyperplasia is a "group of inherited disorders of the adrenal glands, caused by enzyme defects in the synthesis of cortisol (hydrocortisone) and/or aldosterone leading to accumulation of precursors for androgens. Depending on the hormone imbalance, congenital adrenal hyperplasia can be classified as salt-wasting, hypertensive, virilizing, or feminizing. The most common defect is in steroid 21-hydroxylase. Other defects occur in steroid 11-beta-hydroxylase, steroid 17-alpha-hydroxylase, or 3-beta-hydroxysteroid dehydrogenase (3-hydroxysteroid dehydrogenases)".[4] [5][6]
- Biochemistry
Some people inherit defective forms of the enzyme 21-hydroxylase. Because 21-hydroxylase is a key enzyme in the production of the corticosteroids, defects in this enzyme lead to severe physiological effects. The enzyme is required for the production of cortisol, corticosterone, aldosterone and other steroids. Diminished levels of the glucocorticoids induces the anteterior pituitary gland to increase production of ACTH, which in turn increases the levels of progesterone and 17hydroxy-progesterone. High levels of these two hormones increases the levels of androgens, which are derived from them. The pathogenesis can be stopped by the administration of glucocorticoids to complete the feed-back mechanism and halt overproduction of ACTH.
- Manifestations
Aldosterone is the main mineralcorticoid and is produced in the adrenal glands. It plays a critical role in maintaining normal plasma concentrations of sodium; patients with 21-hydroxylase deficiency can suffer hypertension and dehydration, which can lead to shock or death. Increases in the levels of the androgens (testosterone, for example) resulting from the overproduction of progesterone and 17-hydroxy-progesterone lead to the virilization ((masculinization) of those affected. In girls, masculinization of the external genitalia is usually evident at birth. Boys appear normal at birth but sexual precocity soon follows. Accelerated growth and early bone development leads to a shortened stature.
The Nobel Prize and steroid research
Many scientists have won the Nobel Prize for work on these molecules.
The 1927 Nobel Prize in chemistry was awarded to German chemist Heinrich Otto Wieland (1977-1957) "for his investigations of the constitution of the bile acids and related substances" "for the services rendered through his research into the constitution of the sterols and their connection with the vitamins" investigations he began in 1912 on the constitution of bile acids and related substances. He isolated three bile acids and discovered that they had similar structures and were structurally related to cholesterol. This work led to the recognition of the carbon framework of steroids and the study of oxidation processes in living cells.
In 1928, the prize went to Adolf Otto Reinhold Windaus (1876-1959) "for the services rendered through his research into the constitution of the sterols and their connection with the vitamins"[7]
In 1939, Adolf Butenandt (1903-1995) was awarded the The Nobel Prize in Chemistry in 1939 for his work on sex hormones, jointly with Leopold Ruzicka for his work on polymethylenes and higher terpenes In [8]. Butenandt isolated oestrone in pure, crystalline form in 1929 and androsterone in 1931. From androsterone he (as well as Ruzicka, independently of each other) obtained testosterone in 1939. He isolated progesterone from the corpus luteum in 1934.[9]
In 1950, the Nobel Prize for Physiology or Medicine went jointly to Edward Kendall (1886-1972)[10] , Tadeus Reichstein (1897-1976) [11] and Philip Hench (1896-1965) [12] "for their discoveries relating to the hormones of the adrenal cortex, their structure and biological effects"
- "Professor Edward Kendall. You and your collaborators have greatly contributed to the isolation and identification of the cortical hormones, and you have facilitated the artificial production of some of these substances. You have shown conclusively that their biological actions differ all according to their chemical structure.
- "Professor Tadeus Reichstein. To you and your co-workers we owe the first isolation of four active hormones from the adrenal cortex, the first synthesis of one of them, the proof of the steroid nature of said hormones, and numerous details on the structure and properties of these important bodies. In this way the wearisome road to synthesis was smoothed, and new medicaments created.
- "Doctor Philip Hench. Your brilliant investigations in respect of the beneficial effects of pregnancy and jaundice on rheumatoid arthritis have been the starting-point for the famous discovery during «the preceding year» that these diseases and some others are favourably influenced by hormones from the adrenal cortex."
- (address by Professor G. Liljestrand)
In 1969, Derek Barton (1918-1998) was awarded the Nobel Prize in Chemistry fore "contributions to the development of the concept of conformation and its application in chemistry". In 1950, in a short paper in Experientia entitled "The Conformation of the Steroid Nucleus", he had shown that organic molecules in general (and steroid molecules in particular) could be assigned a preferred conformation based upon results accumulated by chemical physicists, in particular by Odd Hassel, with whom he shared the prize .[13]
See also
References
- ↑ Losel RM, Falkenstein ELIS, Feuring MART, Tillmann HC, Rossol-Haseroth KARI, Wehling MART. (2003) Nongenomic Steroid Action: Controversies, Questions, and Answers. Physiological Reviews 83(3):965-1016.
- ↑ What is 21-hydroxylase deficiency? Genetics Home Reference, U.S. National Library of Medicine.
- ↑ Emmelot-Vonk MH et al (2008). "Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial". JAMA 299: 39–52. DOI:10.1001/jama.2007.51. PMID 18167405. Research Blogging.
- ↑ National Library of Medicine. Adrenal Hyperplasia, Congenital. Retrieved on 2008-01-01.
- ↑ Merke DP, Bornstein SR (2005). "Congenital adrenal hyperplasia". Lancet 365 (9477): 2125–36. DOI:10.1016/S0140-6736(05)66736-0. PMID 15964450. Research Blogging.
- ↑ Speiser PW, White PC (2003). "Congenital adrenal hyperplasia". N. Engl. J. Med. 349 (8): 776–88. DOI:10.1056/NEJMra021561. PMID 12930931. Research Blogging.
- ↑ Adolf Otto Reinhold Windaus
- ↑ Leopold Ruzicka
- ↑ Adolf Butenandt
- ↑ Edward Kendall
- ↑ Tadeus Reichstein
- ↑ Philip Hench
- ↑ Derek Barton