Ebola: Difference between revisions
imported>Simon Overduin |
mNo edit summary |
||
| (18 intermediate revisions by 8 users not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{subpages}} | ||
The '''Ebola''' [[virus]] is named after a river and village in the [[Democratic Republic of the Congo]] (formerly [[Zaïre]]) where it was first discovered and isolated in 1976. | {{Taxobox | color=violet | ||
| name = ''Ebola virus | |||
| virus_group = | |||
| familia = ''[[filoviridae]]'' | |||
| genus = ''[[filovirus]]'' | |||
| sero_complex = | |||
| subdivision_ranks = Species | |||
| subdivision = | |||
}} | |||
The '''Ebola''' [[virus]] is named after a river and village in the [[Democratic Republic of the Congo]] (formerly [[Zaïre]]) where it was first discovered and isolated in 1976. The virus causes severe hemhorragic fever and often leads to death. The virus is easily spread so containment methods must be instituted to stop outbreaks; it is listed in the [[Select Agent Program]] as a dangerously contagious human pathogen. | |||
==Viral Characteristics== | ==Viral Characteristics== | ||
The family of the Ebola virus is the ''filoviridae'' family; the viral haemorrhagic fever associated with it consists of ''filoviridae'' and three other families: ''arenaviridae'', ''flaviviridae'', and ''bunyaviridae''. There is only one [[genus]] in the ''filoviridae'' family, ''filovirus'', and of this genus, there are at least four varieties: [[Marburg]] and Ebola Zaïre, Sudan and Reston. An additional strain of Ebola has been identified following an outbreak in [[Uganda]]. Marburg is distinguished by having a slightly different length than the Ebolas when it is purified. The three kinds of Ebola, each named after a unique region where it broke out, can be identified by serologic and genetic sequence differences. The [[genome]] of the ''Filoviridae'' family has ancient connections with two others, ''Paramyxoviridae'' and ''Rhabdoviridae'', which are responsible for [measles]] and [[mumps]], and [[rabies]], respectively; these three share some genetic sequencing and constitute the only viral order, ''Mononegavirales''. | {{TOC|left}} | ||
The family of the Ebola virus is the ''filoviridae'' family; the viral haemorrhagic fever associated with it consists of ''filoviridae'' and three other families: ''arenaviridae'', ''flaviviridae'', and ''bunyaviridae''. There is only one [[genus]] in the ''filoviridae'' family, ''filovirus'', and of this genus, there are at least four varieties: [[Marburg]] and Ebola Zaïre, Sudan and Reston. An additional strain of Ebola has been identified following an outbreak in [[Uganda]]. Marburg is distinguished by having a slightly different length than the Ebolas when it is purified. The three kinds of Ebola, each named after a unique region where it broke out, can be identified by serologic and genetic sequence differences. The [[genome]] of the ''Filoviridae'' family has ancient connections with two others, ''Paramyxoviridae'' and ''Rhabdoviridae'', which are responsible for [[measles]] and [[mumps]], and [[rabies]], respectively; these three share some genetic sequencing and constitute the only viral order, ''Mononegavirales''. | |||
An Ebola virion has an average length of 920 nm, and a diameter of 80 nm. Their structure consists of a helical nucleocapsid, a membrane of 10 nm projections, and the host cell membrane. The molecular composition of Ebola includes single-stranded [[RNA]], seven [[polypeptide|polypeptides]], a [[nucleoprotein]], [[glycoprotein]], [[polymerase]], and other [[protein|proteins]]. A virus attached to a host cell's membrane takes over the cell's protein-producing factories ([[ribosome|ribosomes]]), and causes the creation of large numbers of viruses before the cell is destroyed. Ebola is what is called a Class Four [[pathogen]]: it requires the highest containment facilities; it responds to no treatment or [[vaccine]], causes a high fatality rate in humans, and can be spread from person to person. | An Ebola virion has an average length of 920 nm, and a diameter of 80 nm. Their structure consists of a helical nucleocapsid, a membrane of 10 nm projections, and the host cell membrane. The molecular composition of Ebola includes single-stranded [[RNA]], seven [[polypeptide|polypeptides]], a [[nucleoprotein]], [[glycoprotein]], [[polymerase]], and other [[protein|proteins]]. A virus attached to a host cell's membrane takes over the cell's protein-producing factories ([[ribosome|ribosomes]]), and causes the creation of large numbers of viruses before the cell is destroyed. Ebola is what is called a Class Four [[pathogen]]: it requires the highest containment facilities; it responds to no treatment or [[vaccine]], causes a high fatality rate in humans, and can be spread from person to person. | ||
| Line 10: | Line 20: | ||
===Emergence in Africa=== | ===Emergence in Africa=== | ||
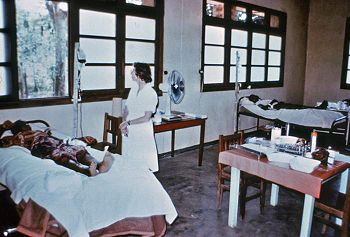
{{Image|1976 ebola outbreak.jpg|right|350px|This photograph showed Dr. Margaret Isaacson as she was tending to the needs of an Ebola patient in a Yambuku, Zaire hospital theatre block that was used as a temporary ICU for Ebola patients during the country’s 1976 outbreak.}} | |||
Three cases of the Ebola virus in Africa caught the attention of medical researchers in the late 1970s. The first outbreak occurred in the [[Democratic Republic of the Congo]] (formerly [[Zaïre]]) in 1976, when a trader, bleeding uncontrollably, arrived at Yambuku hospital along the Ebola river (run by Belgian nuns). Within days, 40% of the nurses who ran the facility were displaying signs of a mysterious infection. For 120 hospital beds, their scanty equipment and medicines included only three needles. In just two weeks, three quarters of the staff had become infected, and 150 had died. When one of the infected nurses traveled to [[Kinshasa]], doctors at the Ngaliema hospital recognized the disease's similarity to the [[Marburg]] virus, which, after emerging around Kitum Cave in eastern [[Kenya]], had been identified in [[Germany]] in 1967. Unlike Marburg, however, this new virus - dubbed Ebola Zaïre - showed no response to a [[serum]] that identified the former filovirus. Although Zaïrean authorities had at first been slow in assessing the potential for viral devastation, the virus soon died down without spreading outside of the country (except through one aid worker who fell ill after he had returned to his native [[United Kingdom]]), and disappeared. | Three cases of the Ebola virus in Africa caught the attention of medical researchers in the late 1970s. The first outbreak occurred in the [[Democratic Republic of the Congo]] (formerly [[Zaïre]]) in 1976, when a trader, bleeding uncontrollably, arrived at Yambuku hospital along the Ebola river (run by Belgian nuns). Within days, 40% of the nurses who ran the facility were displaying signs of a mysterious infection. For 120 hospital beds, their scanty equipment and medicines included only three needles. In just two weeks, three quarters of the staff had become infected, and 150 had died. When one of the infected nurses traveled to [[Kinshasa]], doctors at the Ngaliema hospital recognized the disease's similarity to the [[Marburg]] virus, which, after emerging around Kitum Cave in eastern [[Kenya]], had been identified in [[Germany]] in 1967. Unlike Marburg, however, this new virus - dubbed Ebola Zaïre - showed no response to a [[serum]] that identified the former filovirus. Although Zaïrean authorities had at first been slow in assessing the potential for viral devastation, the virus soon died down without spreading outside of the country (except through one aid worker who fell ill after he had returned to his native [[United Kingdom]]), and disappeared. | ||
Ebola soon struck again, however: the Zaïre case was followed by outbreaks in [[Sudan]] later the same year, and in 1979 - both in a cotton factory. The slight difference between the viruses in each country was only discovered when Ebola Sudan was isolated and carefully observed in foreign laboratories. The death toll of both outbreaks in 1976 was 340, with almost 600 cases reported; Ebola Sudan in 1979 infected only 34 and cost 22 lives. This improved containment was partly a result of quicker action, the presence of appropriate medical supplies and equipment, and the quarantine measures taken by government officials. A further case appears to have occurred in | Ebola soon struck again, however: the Zaïre case was followed by outbreaks in [[Sudan]] later the same year, and in 1979 - both in a cotton factory. The slight difference between the viruses in each country was only discovered when Ebola Sudan was isolated and carefully observed in foreign laboratories. The death toll of both outbreaks in 1976 was 340, with almost 600 cases reported; Ebola Sudan in 1979 infected only 34 and cost 22 lives. This improved containment was partly a result of quicker action, the presence of appropriate medical supplies and equipment, and the quarantine measures taken by government officials. A further case appears to have occurred in [[Côte d'Ivoire]] (Ivory Coast) in 1993; isolated in [[Siena]], [[Italy]], it was labeled as the most dangerous strain up until then. In 2014, a further outbreak occurred in [[West Africa]]. | ||
===Outbreak in North America=== | ===Outbreak in North America, 1989=== | ||
The African outbreaks generated relatively little concern in the Western Hemisphere that the Ebola virus and others like it could become international in scope. Although there were instances in which people managed to carry the virus far beyond its original emergence into more heavily populated centers, the patients were quickly isolated by doctors there who were quickly able to recognize the virus and its significance (the British aid worker, for instance). | The African outbreaks generated relatively little concern in the Western Hemisphere that the Ebola virus and others like it could become international in scope. Although there were instances in which people managed to carry the virus far beyond its original emergence into more heavily populated centers, the patients were quickly isolated by doctors there who were quickly able to recognize the virus and its significance (the British aid worker, for instance). | ||
However, a frightening episode in 1989 brought Ebola much to closer to home in the developed world. Ebola Reston is named not after a location in Africa; rather, it identifies a strain of the virus that managed to break out in the town of [[Reston]], [[Virginia]] - a community from which [[Washington DC]] is visible. This virus was first diagnosed in [[monkey|monkeys]], not humans. The animals were housed at the Reston Primate Quarantine Unit, a monkey-handling facility owned by Hazleton Research Products. The one-story building imported approximately 16,000 wild monkeys annually from the tropics, destined for medical research. In October, a shipment of 100 crab-eating monkeys arrived from Ferlite Farms in the Philippines; two had already died upon arrival. A month later, after many more of the primates had become violently ill, the | However, a frightening episode in 1989 brought Ebola much to closer to home in the developed world. Ebola Reston is named not after a location in Africa; rather, it identifies a strain of the virus that managed to break out in the town of [[Reston]], [[Virginia (U.S. state)|Virginia]] - a community from which [[Washington DC]] is visible. This virus was first diagnosed in [[monkey|monkeys]], not humans. The animals were housed at the Reston Primate Quarantine Unit, a monkey-handling facility owned by Hazleton Research Products. The one-story building imported approximately 16,000 wild monkeys annually from the tropics, destined for medical research. In October, a shipment of 100 crab-eating monkeys arrived from Ferlite Farms in the Philippines; two had already died upon arrival. A month later, after many more of the primates had become violently ill, the United States Army Medical Research Institute of Infectious Diseases in [[Maryland (U.S. state)|Maryland]] had identified an Ebola virus in a sample taken from a dissected primate. Days later, the army undertook a covert depopulation mission in the Reston monkey house, killing all the animals that had not yet died from Ebola Reston of the 500 that were housed there at the time. The building, now abandoned, was sterilized thoroughly after the incident. | ||
Several human workers at the facility were infected with Ebola Reston; strangely, none of them died. It was not until after the event that researchers could conclusively state that the virus for some mysterious reason had no effect on humans, while the reaction in infected monkeys was similar to the symptoms in humans in previous outbreaks. Many other important questions about Ebola Reston have not yet been answered. For instance: How did Ebola end up in a primate host from the Philippines? At the time, there was a civil war going on in the area where Ferlite Farms had captured its monkeys; this prevented investigators from tracking the disease outside of the U.S. (Later serologic studies in the | Several human workers at the facility were infected with Ebola Reston; strangely, none of them died. It was not until after the event that researchers could conclusively state that the virus for some mysterious reason had no effect on humans, while the reaction in infected monkeys was similar to the symptoms in humans in previous outbreaks. Many other important questions about Ebola Reston have not yet been answered. For instance: How did Ebola end up in a primate host from the [[Philippines]]? At the time, there was a civil war going on in the area where Ferlite Farms had captured its monkeys; this prevented investigators from tracking the disease outside of the U.S. (Later serologic studies in the Philippines, and elsewhere in Southeast Asia, suggest that the virus may be a prevalent cause of infection in [[macaque|macaques]].) More important, though, is the debate about whether Ebola Reston, unlike its predecessors, was airborne. The monkeys that became ill were separated by cages, and were contained in a number of separate, sealed rooms, yet nowhere in the building were the animals safe from the disease. | ||
Following the 1989 outbreak, a complete embargo on the import of [[monkey|monkeys]] was initiated, and it lasted one year. The American government decreed that stricter import licenses would be required, with more careful inspections to ensure proper facilities and staff training in the countries from which the animals came. [[Canada]] imposed more stringent quarantine measures for imported monkeys; this has also helped to encourage a trend in medical research of using captive-bred, rather than wild, monkeys. | Following the 1989 outbreak, a complete embargo on the import of [[monkey|monkeys]] was initiated, and it lasted one year. The American government decreed that stricter import licenses would be required, with more careful inspections to ensure proper facilities and staff training in the countries from which the animals came. [[Canada]] imposed more stringent quarantine measures for imported monkeys; this has also helped to encourage a trend in medical research of using captive-bred, rather than wild, monkeys. | ||
===Outbreak in West Africa, 2014=== | |||
Ebola re-emerged in [[West Africa]] in 2014, and by August had claimed over 1,350 lives across [[Guinea]], [[Liberia]], [[Sierra Leone]] and [[Nigeria]]. The outbreak is thought to have originated with a two-year-old girl who died in Guinea in December 2013. Typically, [[sub-Saharan Africa]] sees approximately 500 cases per year.<ref>''BBC News'': '[http://www.bbc.co.uk/news/world-africa-28755033 Ebola: Mapping the outbreak]'. 20th August 2014.</ref> | |||
==Infection== | ==Infection== | ||
| Line 29: | Line 44: | ||
After Ebola enters a host, it finds its way to the bloodstream or tissue fluids, eventually penetrating into a tissue wall, where it begins its multiplication. The [[incubation]] period for Ebola is about five to seven days in patients who contracted the disease via needle, and six to twelve days - at the most, 21 - for person-to-person transmissions. | After Ebola enters a host, it finds its way to the bloodstream or tissue fluids, eventually penetrating into a tissue wall, where it begins its multiplication. The [[incubation]] period for Ebola is about five to seven days in patients who contracted the disease via needle, and six to twelve days - at the most, 21 - for person-to-person transmissions. | ||
The virus, spread through blood vessels, is replicated in organs throughout the body, including the [[liver]], [[lymphatic system|lymphatic]] and some sensory organs, [[kidney|kidneys]], [[ | The virus, spread through blood vessels, is replicated in organs throughout the body, including the [[liver]], [[lymphatic system|lymphatic]] and some sensory organs, [[kidney|kidneys]], [[Ovary (human)|ovaries]] and [[teste|testes]]. Hardest hit are [[liver]] cells and [[macrophage|macrophages]], or the cells of the [[reticuloendothelial system]]. Lesions form within the body, and cause internal and external bleeding at those sites, especially in the [[mucosa]], [[abdomen]], [[pericardium]], and [[vagina]]. [[vascular system|Vascular]] integrity is degraded as cells and [[capillary|capillaries]] leak plasma. The first outward symptoms, which usually appear within ten days of infection, include lethargy, [[fever]], chills, [[headache|headaches]], [[muscle]] aches and a loss of appetite. As the disease progresses, victims experience vomiting, diarrhoea, sore throats, and abdominal and chest pain. [[Skin]] rashes may form, and the patient may bleed externally from injection sites, the mouth, the nose, and any other body orifices. Deliriousness and combativeness eventually give way to mute imperceptivity. In fatal cases, the kidneys and liver fail, and an acute respiratory disorder develops. Patients of this hemorrhagic fever eventually die of intractable [[shock]]. In extreme cases, sufferers of the Ebola virus lives long enough for their bodies to turn virtually to liquid. | ||
Ebola has a human fatality rate of 50-90%. But what of those who survive this terrible death? This, another mysterious aspect of the virus, is answered partly by observations of natural antibodies to Ebola formed in some survivors' bodies. The only protective defense the body provides is a specifically-binding, neutralizing [[antibody]] formed in the lymph glands, where the first viral replication often occurs. (Normally the antibodies are not produced quickly enough to battle the pathogen; even in cases where they do grant endurance to a patient, they are no guarantee against re-infection.) The surviving patient also has the advantage that Ebola is self-limiting when ineffective in a human host (although it has been detected 30 days or more after an infection in semen and in the vitreous of the eye) - and little chance exists of spreading the disease after recovery. | Ebola has a human fatality rate of 50-90%. But what of those who survive this terrible death? This, another mysterious aspect of the virus, is answered partly by observations of natural antibodies to Ebola formed in some survivors' bodies. The only protective defense the body provides is a specifically-binding, neutralizing [[antibody]] formed in the lymph glands, where the first viral replication often occurs. (Normally the antibodies are not produced quickly enough to battle the pathogen; even in cases where they do grant endurance to a patient, they are no guarantee against re-infection.) The surviving patient also has the advantage that Ebola is self-limiting when ineffective in a human host (although it has been detected 30 days or more after an infection in semen and in the vitreous of the eye) - and little chance exists of spreading the disease after recovery. | ||
| Line 35: | Line 50: | ||
==Control== | ==Control== | ||
Diagnosis of the Ebola virus consists of testing, in a high containment laboratory, an inactivated viral specimen in a culture to detect [antigen|antigens]] or [[antibody|antibodies]] that are produced in response to its presence, or by scrutinizing the genetic material. No treatment, however, is effective against the Ebola virus; when an outbreak occurs, experts say that the only thing to do is to try to contain the disease. This includes the use of proper medical equipment and hygienic procedures, including masks, gowns, gloves, and carefully sterilized needles and syringes, the safe disposal of waste and corpses, and patient isolation. These simple requirements can be difficult to obtain in developing nations such as the [[Democratic Republic of the Congo]], where patients may be requested to bring in their own needles, or else share, and where even the washing of hands is sometimes ineffective because running water is not always available. The barrier technique used by hospital personnel in isolating the ill consists of protection of doctors and nurses when caring for the patient, restriction of visitors, removal and incineration of disposable materials from the patient's room, and sterilization of reusable supplies and all hard surfaces with disinfectant. Yet in the African outbreaks, there has been difficulty in rural areas in getting the ill into local hospitals, for their families have a cultural responsibility to stay with sick or dying members; when Ebola patients become ill and see that their health does not improve, they may leave a hospital, for another or for home. | Diagnosis of the Ebola virus consists of testing, in a high containment laboratory, an inactivated viral specimen in a culture to detect [[antigen|antigens]] or [[antibody|antibodies]] that are produced in response to its presence, or by scrutinizing the genetic material. No treatment, however, is effective against the Ebola virus; when an outbreak occurs, experts say that the only thing to do is to try to contain the disease. This includes the use of proper medical equipment and hygienic procedures, including masks, gowns, gloves, and carefully sterilized needles and syringes, the safe disposal of waste and corpses, and patient isolation. These simple requirements can be difficult to obtain in developing nations such as the [[Democratic Republic of the Congo]], where patients may be requested to bring in their own needles, or else share, and where even the washing of hands is sometimes ineffective because running water is not always available. The barrier technique used by hospital personnel in isolating the ill consists of protection of doctors and nurses when caring for the patient, restriction of visitors, removal and incineration of disposable materials from the patient's room, and sterilization of reusable supplies and all hard surfaces with disinfectant. Yet in the African outbreaks, there has been difficulty in rural areas in getting the ill into local hospitals, for their families have a cultural responsibility to stay with sick or dying members; when Ebola patients become ill and see that their health does not improve, they may leave a hospital, for another or for home. | ||
[[Antiviral]] therapies, [[interferon]] and [[ribavirin]] have had no effect on human Ebola. Treatment of infected monkeys with an inhibitor of [[coagulation]] or with [[antisense]] drugs. [[Vaccine]] development has also had success only in monkeys, in whom vaccines for both Ebola and Marburg have been shown to be highly effective. | [[Antiviral]] therapies, [[interferon]] and [[ribavirin]] have had no effect on human Ebola. Treatment of infected monkeys with an inhibitor of [[coagulation]] or with [[antisense]] drugs. [[Vaccine]] development has also had success only in monkeys, in whom vaccines for both Ebola and Marburg have been shown to be highly effective. | ||
| Line 46: | Line 61: | ||
Normally, Ebola has been transmitted through person-to-person contact between hospital care workers or family members, and also through the reuse of hypodermic needles. Sexual contact has also been identified as a means of transmission. The potential for airborne travel, however, is significant. Ebola's use of [[RNA]] rather than [[DNA]] as its genetic material confers on it an advantage: mutations in the copying process abound. The high incidence of error in Ebola's synthesis is a result of the lack of mechanisms in the single-stranded RNA to correct faults. Only a very small, unidentifiable alteration in a section of the Ebola Reston genome allowed it to spread differently than Ebola Zaïre and Sudan. Whereas the primary route of infection in earlier epidemics was through needles, syringes, and otherwise through close contact with the infected, Ebola Reston demonstrated greater ability for aerosol infection. Such airborne transmission poses greater challenges for containment. With densely populated communities and substandard medical facilities in countries like the [[Democratic Republic of the Congo]] and [[Sudan]], it may be very difficult to prevent the arrival of someone at a hospital with vague, flu-like symptoms from turning into a country-wide epidemic.� | Normally, Ebola has been transmitted through person-to-person contact between hospital care workers or family members, and also through the reuse of hypodermic needles. Sexual contact has also been identified as a means of transmission. The potential for airborne travel, however, is significant. Ebola's use of [[RNA]] rather than [[DNA]] as its genetic material confers on it an advantage: mutations in the copying process abound. The high incidence of error in Ebola's synthesis is a result of the lack of mechanisms in the single-stranded RNA to correct faults. Only a very small, unidentifiable alteration in a section of the Ebola Reston genome allowed it to spread differently than Ebola Zaïre and Sudan. Whereas the primary route of infection in earlier epidemics was through needles, syringes, and otherwise through close contact with the infected, Ebola Reston demonstrated greater ability for aerosol infection. Such airborne transmission poses greater challenges for containment. With densely populated communities and substandard medical facilities in countries like the [[Democratic Republic of the Congo]] and [[Sudan]], it may be very difficult to prevent the arrival of someone at a hospital with vague, flu-like symptoms from turning into a country-wide epidemic.� | ||
==Footnotes== | |||
{{reflist|2}} | |||
==References== | ==References== | ||
* Preston, Richard. ''The Hot Zone''. New York: Random House, 1994. | * Preston, Richard. ''The Hot Zone''. New York: Random House, 1994.[[Category:Suggestion Bot Tag]] | ||
[[Category: | |||
Latest revision as of 16:01, 9 August 2024
| Ebola virus | ||||
|---|---|---|---|---|
| Scientific classification | ||||
|
The Ebola virus is named after a river and village in the Democratic Republic of the Congo (formerly Zaïre) where it was first discovered and isolated in 1976. The virus causes severe hemhorragic fever and often leads to death. The virus is easily spread so containment methods must be instituted to stop outbreaks; it is listed in the Select Agent Program as a dangerously contagious human pathogen.
Viral Characteristics
The family of the Ebola virus is the filoviridae family; the viral haemorrhagic fever associated with it consists of filoviridae and three other families: arenaviridae, flaviviridae, and bunyaviridae. There is only one genus in the filoviridae family, filovirus, and of this genus, there are at least four varieties: Marburg and Ebola Zaïre, Sudan and Reston. An additional strain of Ebola has been identified following an outbreak in Uganda. Marburg is distinguished by having a slightly different length than the Ebolas when it is purified. The three kinds of Ebola, each named after a unique region where it broke out, can be identified by serologic and genetic sequence differences. The genome of the Filoviridae family has ancient connections with two others, Paramyxoviridae and Rhabdoviridae, which are responsible for measles and mumps, and rabies, respectively; these three share some genetic sequencing and constitute the only viral order, Mononegavirales.
An Ebola virion has an average length of 920 nm, and a diameter of 80 nm. Their structure consists of a helical nucleocapsid, a membrane of 10 nm projections, and the host cell membrane. The molecular composition of Ebola includes single-stranded RNA, seven polypeptides, a nucleoprotein, glycoprotein, polymerase, and other proteins. A virus attached to a host cell's membrane takes over the cell's protein-producing factories (ribosomes), and causes the creation of large numbers of viruses before the cell is destroyed. Ebola is what is called a Class Four pathogen: it requires the highest containment facilities; it responds to no treatment or vaccine, causes a high fatality rate in humans, and can be spread from person to person.
Origins
Emergence in Africa
Three cases of the Ebola virus in Africa caught the attention of medical researchers in the late 1970s. The first outbreak occurred in the Democratic Republic of the Congo (formerly Zaïre) in 1976, when a trader, bleeding uncontrollably, arrived at Yambuku hospital along the Ebola river (run by Belgian nuns). Within days, 40% of the nurses who ran the facility were displaying signs of a mysterious infection. For 120 hospital beds, their scanty equipment and medicines included only three needles. In just two weeks, three quarters of the staff had become infected, and 150 had died. When one of the infected nurses traveled to Kinshasa, doctors at the Ngaliema hospital recognized the disease's similarity to the Marburg virus, which, after emerging around Kitum Cave in eastern Kenya, had been identified in Germany in 1967. Unlike Marburg, however, this new virus - dubbed Ebola Zaïre - showed no response to a serum that identified the former filovirus. Although Zaïrean authorities had at first been slow in assessing the potential for viral devastation, the virus soon died down without spreading outside of the country (except through one aid worker who fell ill after he had returned to his native United Kingdom), and disappeared.
Ebola soon struck again, however: the Zaïre case was followed by outbreaks in Sudan later the same year, and in 1979 - both in a cotton factory. The slight difference between the viruses in each country was only discovered when Ebola Sudan was isolated and carefully observed in foreign laboratories. The death toll of both outbreaks in 1976 was 340, with almost 600 cases reported; Ebola Sudan in 1979 infected only 34 and cost 22 lives. This improved containment was partly a result of quicker action, the presence of appropriate medical supplies and equipment, and the quarantine measures taken by government officials. A further case appears to have occurred in Côte d'Ivoire (Ivory Coast) in 1993; isolated in Siena, Italy, it was labeled as the most dangerous strain up until then. In 2014, a further outbreak occurred in West Africa.
Outbreak in North America, 1989
The African outbreaks generated relatively little concern in the Western Hemisphere that the Ebola virus and others like it could become international in scope. Although there were instances in which people managed to carry the virus far beyond its original emergence into more heavily populated centers, the patients were quickly isolated by doctors there who were quickly able to recognize the virus and its significance (the British aid worker, for instance).
However, a frightening episode in 1989 brought Ebola much to closer to home in the developed world. Ebola Reston is named not after a location in Africa; rather, it identifies a strain of the virus that managed to break out in the town of Reston, Virginia - a community from which Washington DC is visible. This virus was first diagnosed in monkeys, not humans. The animals were housed at the Reston Primate Quarantine Unit, a monkey-handling facility owned by Hazleton Research Products. The one-story building imported approximately 16,000 wild monkeys annually from the tropics, destined for medical research. In October, a shipment of 100 crab-eating monkeys arrived from Ferlite Farms in the Philippines; two had already died upon arrival. A month later, after many more of the primates had become violently ill, the United States Army Medical Research Institute of Infectious Diseases in Maryland had identified an Ebola virus in a sample taken from a dissected primate. Days later, the army undertook a covert depopulation mission in the Reston monkey house, killing all the animals that had not yet died from Ebola Reston of the 500 that were housed there at the time. The building, now abandoned, was sterilized thoroughly after the incident.
Several human workers at the facility were infected with Ebola Reston; strangely, none of them died. It was not until after the event that researchers could conclusively state that the virus for some mysterious reason had no effect on humans, while the reaction in infected monkeys was similar to the symptoms in humans in previous outbreaks. Many other important questions about Ebola Reston have not yet been answered. For instance: How did Ebola end up in a primate host from the Philippines? At the time, there was a civil war going on in the area where Ferlite Farms had captured its monkeys; this prevented investigators from tracking the disease outside of the U.S. (Later serologic studies in the Philippines, and elsewhere in Southeast Asia, suggest that the virus may be a prevalent cause of infection in macaques.) More important, though, is the debate about whether Ebola Reston, unlike its predecessors, was airborne. The monkeys that became ill were separated by cages, and were contained in a number of separate, sealed rooms, yet nowhere in the building were the animals safe from the disease.
Following the 1989 outbreak, a complete embargo on the import of monkeys was initiated, and it lasted one year. The American government decreed that stricter import licenses would be required, with more careful inspections to ensure proper facilities and staff training in the countries from which the animals came. Canada imposed more stringent quarantine measures for imported monkeys; this has also helped to encourage a trend in medical research of using captive-bred, rather than wild, monkeys.
Outbreak in West Africa, 2014
Ebola re-emerged in West Africa in 2014, and by August had claimed over 1,350 lives across Guinea, Liberia, Sierra Leone and Nigeria. The outbreak is thought to have originated with a two-year-old girl who died in Guinea in December 2013. Typically, sub-Saharan Africa sees approximately 500 cases per year.[1]
Infection
After Ebola enters a host, it finds its way to the bloodstream or tissue fluids, eventually penetrating into a tissue wall, where it begins its multiplication. The incubation period for Ebola is about five to seven days in patients who contracted the disease via needle, and six to twelve days - at the most, 21 - for person-to-person transmissions.
The virus, spread through blood vessels, is replicated in organs throughout the body, including the liver, lymphatic and some sensory organs, kidneys, ovaries and testes. Hardest hit are liver cells and macrophages, or the cells of the reticuloendothelial system. Lesions form within the body, and cause internal and external bleeding at those sites, especially in the mucosa, abdomen, pericardium, and vagina. Vascular integrity is degraded as cells and capillaries leak plasma. The first outward symptoms, which usually appear within ten days of infection, include lethargy, fever, chills, headaches, muscle aches and a loss of appetite. As the disease progresses, victims experience vomiting, diarrhoea, sore throats, and abdominal and chest pain. Skin rashes may form, and the patient may bleed externally from injection sites, the mouth, the nose, and any other body orifices. Deliriousness and combativeness eventually give way to mute imperceptivity. In fatal cases, the kidneys and liver fail, and an acute respiratory disorder develops. Patients of this hemorrhagic fever eventually die of intractable shock. In extreme cases, sufferers of the Ebola virus lives long enough for their bodies to turn virtually to liquid.
Ebola has a human fatality rate of 50-90%. But what of those who survive this terrible death? This, another mysterious aspect of the virus, is answered partly by observations of natural antibodies to Ebola formed in some survivors' bodies. The only protective defense the body provides is a specifically-binding, neutralizing antibody formed in the lymph glands, where the first viral replication often occurs. (Normally the antibodies are not produced quickly enough to battle the pathogen; even in cases where they do grant endurance to a patient, they are no guarantee against re-infection.) The surviving patient also has the advantage that Ebola is self-limiting when ineffective in a human host (although it has been detected 30 days or more after an infection in semen and in the vitreous of the eye) - and little chance exists of spreading the disease after recovery.
Control
Diagnosis of the Ebola virus consists of testing, in a high containment laboratory, an inactivated viral specimen in a culture to detect antigens or antibodies that are produced in response to its presence, or by scrutinizing the genetic material. No treatment, however, is effective against the Ebola virus; when an outbreak occurs, experts say that the only thing to do is to try to contain the disease. This includes the use of proper medical equipment and hygienic procedures, including masks, gowns, gloves, and carefully sterilized needles and syringes, the safe disposal of waste and corpses, and patient isolation. These simple requirements can be difficult to obtain in developing nations such as the Democratic Republic of the Congo, where patients may be requested to bring in their own needles, or else share, and where even the washing of hands is sometimes ineffective because running water is not always available. The barrier technique used by hospital personnel in isolating the ill consists of protection of doctors and nurses when caring for the patient, restriction of visitors, removal and incineration of disposable materials from the patient's room, and sterilization of reusable supplies and all hard surfaces with disinfectant. Yet in the African outbreaks, there has been difficulty in rural areas in getting the ill into local hospitals, for their families have a cultural responsibility to stay with sick or dying members; when Ebola patients become ill and see that their health does not improve, they may leave a hospital, for another or for home.
Antiviral therapies, interferon and ribavirin have had no effect on human Ebola. Treatment of infected monkeys with an inhibitor of coagulation or with antisense drugs. Vaccine development has also had success only in monkeys, in whom vaccines for both Ebola and Marburg have been shown to be highly effective.
Transmission
The source, or sources, of the Ebola virus have continually eluded researchers. Viral host animals typically include insects, rodents, and primates. Monkeys were clearly not carriers of the Ebola Reston virus, for they had no resistance to it - but this theory may be inapplicable to other strains of the disease. Bats have been considered (both for Ebola and Marburg), because both the bat-filled Kitum Cave and the Sudanese cotton factory - the roof of which provides a home for thousands of bats - were sites of infection in humans.
Human exposure to these hosts and their habitats increases with a number of ecological factors related to the destruction and alteration of this habitat. These changes both make it easier for a human population to be infected, and for the virus to spread through populations of humans. Such factors include uncontrolled urbanization and the intrusion of domestic animals into tropical forests; deforestation and the creation of new boundaries between forests, and farms and transportation routes; primitive irrigation systems and dams, creating places of standing water; new routes for migrating birds because of water pollution and deforestation; more long-distance livestock transportation; and more long-distance air travel. Thus trends in increasing human globalization also lead to a greater vulnerability to epidemics of Ebola and other viruses.
Normally, Ebola has been transmitted through person-to-person contact between hospital care workers or family members, and also through the reuse of hypodermic needles. Sexual contact has also been identified as a means of transmission. The potential for airborne travel, however, is significant. Ebola's use of RNA rather than DNA as its genetic material confers on it an advantage: mutations in the copying process abound. The high incidence of error in Ebola's synthesis is a result of the lack of mechanisms in the single-stranded RNA to correct faults. Only a very small, unidentifiable alteration in a section of the Ebola Reston genome allowed it to spread differently than Ebola Zaïre and Sudan. Whereas the primary route of infection in earlier epidemics was through needles, syringes, and otherwise through close contact with the infected, Ebola Reston demonstrated greater ability for aerosol infection. Such airborne transmission poses greater challenges for containment. With densely populated communities and substandard medical facilities in countries like the Democratic Republic of the Congo and Sudan, it may be very difficult to prevent the arrival of someone at a hospital with vague, flu-like symptoms from turning into a country-wide epidemic.�
Footnotes
- ↑ BBC News: 'Ebola: Mapping the outbreak'. 20th August 2014.
References
- Preston, Richard. The Hot Zone. New York: Random House, 1994.