NADPH: Difference between revisions
Jump to navigation
Jump to search

imported>David E. Volk m (typo) |
mNo edit summary |
||
| (One intermediate revision by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
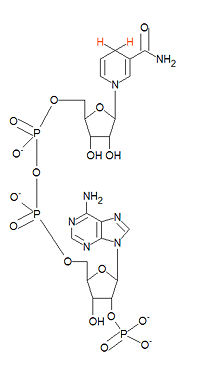
{{Image|Nicotinamide adenine dinucleotide phosphate.jpg|right|200px|Structure of NADPH. NADH differs only by the absence of the lowest phosphate group.}} | |||
'''NADPH''', the reduced from of '''nicotinamide adenine dinucleotide phosphate''', serves as a major reducing agent in biological systems. The oxided form is abbreviated as '''NADP<sup>+</sup>'''. NADPH is a hydride ion donor. Although NADPH and [[NADH]] differ by only the presence of the 2'-phosphate group in NADPH (bottome of structure), their chemistries are drastically different. It is generated by the malate enzyme, photosystems, and in the [[pentose phosphate pathway]]. | '''NADPH''', the reduced from of '''nicotinamide adenine dinucleotide phosphate''', serves as a major reducing agent in biological systems. The oxided form is abbreviated as '''NADP<sup>+</sup>'''. NADPH is a hydride ion donor. Although NADPH and [[NADH]] differ by only the presence of the 2'-phosphate group in NADPH (bottome of structure), their chemistries are drastically different. It is generated by the malate enzyme, photosystems, and in the [[pentose phosphate pathway]].[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 11:00, 22 September 2024
NADPH, the reduced from of nicotinamide adenine dinucleotide phosphate, serves as a major reducing agent in biological systems. The oxided form is abbreviated as NADP+. NADPH is a hydride ion donor. Although NADPH and NADH differ by only the presence of the 2'-phosphate group in NADPH (bottome of structure), their chemistries are drastically different. It is generated by the malate enzyme, photosystems, and in the pentose phosphate pathway.