Efavirenz: Difference between revisions
imported>David E. Volk (chem infobox) |
mNo edit summary |
||
| (6 intermediate revisions by 3 users not shown) | |||
| Line 3: | Line 3: | ||
{{Chem infobox | {{Chem infobox | ||
|align=right | |align=right | ||
|image= | |image= {{Image|Efavirenz structure.jpg|center|200px|}} | ||
|width= | |width=200px | ||
|molname=efavirenz | |molname=efavirenz | ||
|synonyms= Stocrin®, Sustiva® | |synonyms= Stocrin®, Sustiva® | ||
| Line 19: | Line 19: | ||
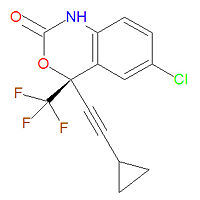
'''Efavirenz''' ('''EFV''') is a non-nucleoside reverse transcriptase inhibitor (NNRTI) and is used as part of [[highly active antiretroviral therapy]] (HAART) for the treatment [[HIV]]-1. It is a synthetic purine derivative similar to [[zidovudine]], [[zalcitabine]], and [[stavudine]]. Efavirenz and [[lamivudine]] with either [[zidovudine]] or [[tenofovir]] is the preferred NNRTI-based regimen for HIV infections not previously treated. It is also used in combination with other anti-HIV drugs in expanded prophylactic treatment upon possible HIV exposure. It is sold under the brand names '''Sustiva'''® and '''Stocrin'''®. Fetal harm, central nervous system symptoms and psychiatric symptoms have been reported with this drug. | '''Efavirenz''' ('''EFV''') is a non-nucleoside reverse transcriptase inhibitor (NNRTI) and is used as part of [[highly active antiretroviral therapy]] (HAART) for the treatment [[HIV]]-1. It is a synthetic purine derivative similar to [[zidovudine]], [[zalcitabine]], and [[stavudine]]. Efavirenz and [[lamivudine]] with either [[zidovudine]] or [[tenofovir]] is the preferred NNRTI-based regimen for HIV infections not previously treated. It is also used in combination with other anti-HIV drugs in expanded prophylactic treatment upon possible HIV exposure. It is sold under the brand names '''Sustiva'''® and '''Stocrin'''®. Fetal harm, central nervous system symptoms and psychiatric symptoms have been reported with this drug. | ||
Its IUPAC chemical name is (4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluoromethyl)-1H-3,1-benzoxazin-2-one and its chemical formula is C<sub>14</sub>H<sub>9</sub>ClF<sub>3</sub>NO<sub>2</sub> (MW = ). | Its IUPAC chemical name is (4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluoromethyl)-1H-3,1-benzoxazin-2-one and its chemical formula is C<sub>14</sub>H<sub>9</sub>ClF<sub>3</sub>NO<sub>2</sub> (MW = 315.6750 g/mol). | ||
== Drug interactions == | == Drug interactions == | ||
| Line 27: | Line 26: | ||
Efavirenz may increase the toxicity of ergot derivatives, including [[dihydroergotamine]], [[dihydroergotoxine]], [[ergotamine]], [[methylergonovine]] and [[methysergide]] and some [[statin]] drugs, including [[atorvastatin]], [[lovastatin]] and [[simavstatin]]. An increased risk of cardiotoxicity and [[arrhythmia]]s occurs when efavirenz is taken with [[astemizole]] or [[cisapride]] or [[terfenadine]]. Increased toxicity of [[benzodiazepine]] is noted when taken with [[alprazolam]], [[midazolam]] or [[triazolam]]. | Efavirenz may increase the toxicity of ergot derivatives, including [[dihydroergotamine]], [[dihydroergotoxine]], [[ergotamine]], [[methylergonovine]] and [[methysergide]] and some [[statin]] drugs, including [[atorvastatin]], [[lovastatin]] and [[simavstatin]]. An increased risk of cardiotoxicity and [[arrhythmia]]s occurs when efavirenz is taken with [[astemizole]] or [[cisapride]] or [[terfenadine]]. Increased toxicity of [[benzodiazepine]] is noted when taken with [[alprazolam]], [[midazolam]] or [[triazolam]]. | ||
== External Links == | == External Links == | ||
{{CZMed}}[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 12:00, 10 August 2024
|
| |||||||
| efavirenz | |||||||
| |||||||
| Uses: | HIV/AIDS | ||||||
| Properties: | RT inhibitor | ||||||
| Hazards: | see drug interactions | ||||||
| |||||||
Efavirenz (EFV) is a non-nucleoside reverse transcriptase inhibitor (NNRTI) and is used as part of highly active antiretroviral therapy (HAART) for the treatment HIV-1. It is a synthetic purine derivative similar to zidovudine, zalcitabine, and stavudine. Efavirenz and lamivudine with either zidovudine or tenofovir is the preferred NNRTI-based regimen for HIV infections not previously treated. It is also used in combination with other anti-HIV drugs in expanded prophylactic treatment upon possible HIV exposure. It is sold under the brand names Sustiva® and Stocrin®. Fetal harm, central nervous system symptoms and psychiatric symptoms have been reported with this drug.
Its IUPAC chemical name is (4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluoromethyl)-1H-3,1-benzoxazin-2-one and its chemical formula is C14H9ClF3NO2 (MW = 315.6750 g/mol).
Drug interactions
St. John's Wort and saquinavir decrease the antiretroviral effects of efavirenz, and efavirenz decreases the levels and/or effects of atazanavir, clarithromycin, cyclosporin, indinavir, methadone and voriconazole.
Efavirenz may increase the toxicity of ergot derivatives, including dihydroergotamine, dihydroergotoxine, ergotamine, methylergonovine and methysergide and some statin drugs, including atorvastatin, lovastatin and simavstatin. An increased risk of cardiotoxicity and arrhythmias occurs when efavirenz is taken with astemizole or cisapride or terfenadine. Increased toxicity of benzodiazepine is noted when taken with alprazolam, midazolam or triazolam.
External Links
The most up-to-date information about Efavirenz and other drugs can be found at the following sites.
- Efavirenz - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Efavirenz - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Efavirenz - Detailed information from DrugBank.