Pregabalin: Difference between revisions
imported>Caesar Schinas m (Bot: Replacing medical templates with CZMed) |
mNo edit summary |
||
| (6 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
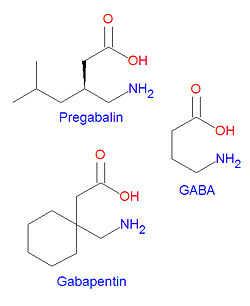
{{Image|Pregabalin and related compounds.jpg|right|250px|Pregabalin and gabapentin are structurally similar to GABA.}} | |||
'''Pregabalin''' is structurally similar to [[gabapentin]]. It is | '''Pregabalin''' (marketed as '''Lyrica'''®) is structurally similar to [[gabapentin]]. It is an analog of [[gamma-aminobutyric acid]] (GABA), the major inhibitory [[neurotransmitter]] in the [[central nervous system]]. Although pregabaliln does not act on GABA receptors, it may increase the "density of GABA transporter protein and increases the rate of functional GABA transport".<ref name="DailyMed">{{DailyMed}}</ref> It is approved by the FDA for neuropathic pain associated with [[diabetic neuropathy|diabetic peripheral neuropathy]], postherpetic neuralgia adjunctive therapy for adult patients with partial onset [[seizure]]s, and [[fibromyalgia]].<ref name="DailyMed"/> | ||
</ref> It is approved by the FDA for neuropathic pain associated with [[diabetic neuropathy|diabetic peripheral neuropathy]], postherpetic neuralgia adjunctive therapy for adult patients with partial onset [[seizure]]s, and [[fibromyalgia]].<ref name="DailyMed" | |||
== | == Chemistry == | ||
The IUPAC chemical name for pregabalin is (S)-(+)-3-(aminomethyl)-5-methylhexanoic acid. Its chemical formula is C<sub>8</sub>H<sub>17</sub>NO<sub>2</sub> giving it a molecular mass of 159.23 g/mol. It is both an [[amine]] and a [[carboxylic acid]]. | The IUPAC chemical name for pregabalin is (S)-(+)-3-(aminomethyl)-5-methylhexanoic acid. Its chemical formula is C<sub>8</sub>H<sub>17</sub>NO<sub>2</sub> giving it a molecular mass of 159.23 g/mol. It is both an [[amine]] and a [[carboxylic acid]]. | ||
==Clinical uses== | |||
Pregabilin is "likely to be beneficial" in the treatment of generalized [[anxiety]] according to Clinical Evidence.<ref name="pmid19450347">{{cite journal| author=Gale CK, Millichamp J| title=Generalised anxiety disorder. | journal=Clin Evid (Online) | year= 2007 | volume= 2007 | issue= | pages= | pmid=19450347 | doi= | pmc=PMC2943796 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19450347 }} </ref> | |||
==Drug toxicity== | |||
At maximum doses of 600 mg per day, [[drug toxicity]] from pregabalin may include reduced cognitive function.<ref name="pmid20194915">{{cite journal| author=Salinsky M, Storzbach D, Munoz S| title=Cognitive effects of pregabalin in healthy volunteers: a double-blind, placebo-controlled trial. | journal=Neurology | year= 2010 | volume= 74 | issue= 9 | pages= 755-61 | pmid=20194915 | |||
| url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=clinical.uthscsa.edu/cite&retmode=ref&cmd=prlinks&id=20194915 | doi=10.1212/WNL.0b013e3181d25b34 }} </ref> | |||
== External links == | == External links == | ||
{{CZMed}} | {{CZMed}} | ||
==References== | ==References== | ||
<references/> | <references/>[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 16:00, 6 October 2024
Pregabalin (marketed as Lyrica®) is structurally similar to gabapentin. It is an analog of gamma-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the central nervous system. Although pregabaliln does not act on GABA receptors, it may increase the "density of GABA transporter protein and increases the rate of functional GABA transport".[1] It is approved by the FDA for neuropathic pain associated with diabetic peripheral neuropathy, postherpetic neuralgia adjunctive therapy for adult patients with partial onset seizures, and fibromyalgia.[1]
Chemistry
The IUPAC chemical name for pregabalin is (S)-(+)-3-(aminomethyl)-5-methylhexanoic acid. Its chemical formula is C8H17NO2 giving it a molecular mass of 159.23 g/mol. It is both an amine and a carboxylic acid.
Clinical uses
Pregabilin is "likely to be beneficial" in the treatment of generalized anxiety according to Clinical Evidence.[2]
Drug toxicity
At maximum doses of 600 mg per day, drug toxicity from pregabalin may include reduced cognitive function.[3]
External links
The most up-to-date information about Pregabalin and other drugs can be found at the following sites.
- Pregabalin - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Pregabalin - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Pregabalin - Detailed information from DrugBank.
References
- ↑ 1.0 1.1 Pregabalin - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- ↑ Gale CK, Millichamp J (2007). "Generalised anxiety disorder.". Clin Evid (Online) 2007. PMID 19450347. PMC PMC2943796. [e]
- ↑ Salinsky M, Storzbach D, Munoz S (2010). "Cognitive effects of pregabalin in healthy volunteers: a double-blind, placebo-controlled trial.". Neurology 74 (9): 755-61. DOI:10.1212/WNL.0b013e3181d25b34. PMID 20194915. Research Blogging.