Torcetrapib: Difference between revisions
imported>David E. Volk (new stub with pix) |
mNo edit summary |
||
| (5 intermediate revisions by 2 users not shown) | |||
| Line 9: | Line 9: | ||
|uses=cardiac medication | |uses=cardiac medication | ||
|properties= | |properties= | ||
|hazards= | |hazards=increased death and cardiac events | ||
|iupac= see chemistry section | |iupac= see chemistry section | ||
|casnumber= 262352-17-0 | |casnumber= 262352-17-0 | ||
}} | }} | ||
Torcetrapib | Torcetrapib was a drug in development to be used to modulate cholesterol levels by its inhibition of [[cholesteryl ester transferase protein]] (CETP). | ||
Due to safety concerns, Pfizer announced its withdrawal of torcetrapib from development<ref> url=http://www.pfizer.com/pfizer/download/investors/financial/8k_1202_06.pdf</ref> Although torcetrapib quickly raised HDL [[cholesterol]] in the ILLUMINATE study, patients receiving both torcetrapib and [[atorvastatin]] has increased incidence of adverse cardiac events and death compared to the control group only receiving atorvastatin. Similar medications include [[anacetrapib]] and [[dalcetrapib]]. | |||
== Chemistry == | == Chemistry == | ||
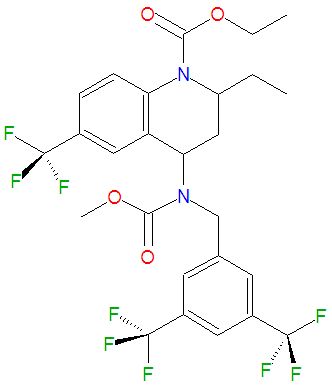
The chemical name of torcetrapib is (2R,4S)-4-[(3,5-Bis-trifluoromethylbenzyl)methoxycarbonylamino]-2-ethyl-6-trifluoromethyl-3,4-dihydro-2H-quinoline-1-carboxylic acid ethyl ester. | The chemical name of torcetrapib is (2R,4S)-4-[(3,5-Bis-trifluoromethylbenzyl)methoxycarbonylamino]-2-ethyl-6-trifluoromethyl-3,4-dihydro-2H-quinoline-1-carboxylic acid ethyl ester. The drug has a formula weight of 600.47 g/mol and is registered under the CAS number 262352-17-0. | ||
== References == | |||
<references/> | |||
[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 16:00, 29 October 2024
|
| |||||||
| torcetrapib | |||||||
| |||||||
| Uses: | cardiac medication | ||||||
| Properties: | |||||||
| Hazards: | increased death and cardiac events | ||||||
| |||||||
Torcetrapib was a drug in development to be used to modulate cholesterol levels by its inhibition of cholesteryl ester transferase protein (CETP).
Due to safety concerns, Pfizer announced its withdrawal of torcetrapib from development[1] Although torcetrapib quickly raised HDL cholesterol in the ILLUMINATE study, patients receiving both torcetrapib and atorvastatin has increased incidence of adverse cardiac events and death compared to the control group only receiving atorvastatin. Similar medications include anacetrapib and dalcetrapib.
Chemistry
The chemical name of torcetrapib is (2R,4S)-4-[(3,5-Bis-trifluoromethylbenzyl)methoxycarbonylamino]-2-ethyl-6-trifluoromethyl-3,4-dihydro-2H-quinoline-1-carboxylic acid ethyl ester. The drug has a formula weight of 600.47 g/mol and is registered under the CAS number 262352-17-0.