User:Milton Beychok/Sandbox: Difference between revisions
imported>Milton Beychok mNo edit summary |
imported>Milton Beychok mNo edit summary |
||
| Line 1: | Line 1: | ||
{{Image|BTX.png|right|350px|Structural diagrams of the BTX aromatic hydrocarbons.}} | {{Image|BTX.png|right|350px|Structural diagrams of the BTX aromatic hydrocarbons.}} | ||
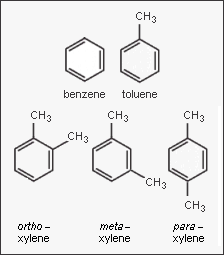
In the petroleum and petrochemical industries, the acronym '''BTX''' refers to mixtures of the aromatic hydrocarbons benzene, toluene, and the three xylene isomers. The xylene isomers are distinguished by the designations ''ortho'' – or ''o'' –, ''meta'' – or ''m'' –, and ''para'' – or ''p'' – as indicated in the adjacent diagram. If ethylbenzene is included, the mixture is sometimes referred to as '''BTEX'''. | In the petroleum and petrochemical industries, the acronym '''BTX''' refers to mixtures of the aromatic hydrocarbons benzene, toluene, and the three xylene isomers. The xylene isomers are distinguished by the designations ''ortho'' – or ''o'' –, ''meta'' – or ''m'' –, and ''para'' – or ''p'' – as indicated in the adjacent diagram. If ethylbenzene is included, the mixture is sometimes referred to as '''BTEX'''. | ||
The BTX aromatics are very important petrochemical materials. The world wide demand for these hydrocarbons is steadily increasing. The demand for xylenes, particularly ''para'' – xylene, has increased in proportion to the increase in demand for polyester fibers and film. Benzene is a highly valuable product for use as a chemical raw material. Toluene is also a valuable petrochemical for use as a solvent and intermediate in chemical manufacturing processes and as a high octane gasoline component. | The BTX aromatics are very important petrochemical materials. The world wide demand for these hydrocarbons is steadily increasing. The demand for xylenes, particularly ''para'' – xylene, has increased in proportion to the increase in demand for polyester fibers and film. Benzene is a highly valuable product for use as a chemical raw material. Toluene is also a valuable petrochemical for use as a solvent and intermediate in chemical manufacturing processes and as a high octane gasoline component.<ref name=BTX-Chain>[http://www1.eere.energy.gov/manufacturing/industries_technologies/chemicals/pdfs/profile_chap4.pdf The BTX Chain: Benzene, Toluene, Xylene]. Chapter 4 of the DOE's Office of Energy Efficiency and Renewable Energy (EERE) report entitled [http://www1.eere.energy.gov/manufacturing/industries_technologies/chemicals/pdfs/profile_full.pdf "Energy and Environmental Profile of the U.S. Chemical Industry" of May 2000.]</ref><ref name=Siemans>[http://www.industry.usa.siemens.com/automation/us/en/process-instrumentation-and-analytics/process-analytics/pa-case-studies/Documents/CS_AROMATICS_EN.pdf Use of Process Analytics in Aromatics (BTX and phenol) production plants]. Case Study, August 2008. (scroll down to "Aromatics" section.</ref><ref name=Chem-Systems>[http://www.chemsystems.com/about/cs/news/items/PERP%200607_6_BenzeneToluene.cfm Benzene/Toluene]. Introduction to a ChemSystems report, 2009.</ref><ref name=TrecanniEncyclopedia>[http://www.treccani.it/export/sites/default/Portale/sito/altre_aree/Tecnologia_e_Scienze_applicate/enciclopedia/inglese/inglese_vol_2/591-614_ING3.pdf 10.6 Aromatics], Online Italian [http://www.treccani.it/export/sites/default/Portale/sito/altre_aree/Tecnologia_e_Scienze_applicate/enciclopedia/inglese/inglese_vol_2/idro_vol_2_I-XXXVI_eng.pdf <i>Encyclopedia of Hydrocarbons</i>], Istituto della Enciclopedia Italiana, Volume II, 2006, pages 603-605.</ref> | ||
==Properties of the BTX hydrocarbons== | ==Properties of the BTX hydrocarbons== | ||
| Line 19: | Line 8: | ||
The table below lists some of the properties of the BTX aromatic hydrocarbons, all of which are liquids at typical room conditions: | The table below lists some of the properties of the BTX aromatic hydrocarbons, all of which are liquids at typical room conditions: | ||
{|class=wikitable align= | {|class=wikitable align= | ||
|+Properties | |+Properties<ref name=CRC-Hdbk>{{cite book|author=Robert C. Weast (Editor)|title=Handbook of Chemistry and Physics|edition=56th Edition|publisher=CRC Press|year=1975|id=ISBN 0-87819-455-X}}</ref> | ||
! !!benzene!!toluene!!ethylbenzene!!''p'' - xylene!!''m'' - xylene!!''o'' - xylene | ! !!benzene!!toluene!!ethylbenzene!!''p'' - xylene!!''m'' - xylene!!''o'' - xylene | ||
|- align=center | |- align=center | ||
| Line 36: | Line 25: | ||
| | | | ||
{|class=wikitable align="right" | {|class=wikitable align="right" | ||
|+BTX content of Pyrolysis Gasoline and Reformate | |+BTX content of Pyrolysis Gasoline and Reformate<ref name=Chem-Systems/> | ||
!rowspan=2| !!colspan=2|Pyrolysis!!colspan=2|Reformate | !rowspan=2| !!colspan=2|Pyrolysis!!colspan=2|Reformate | ||
|- | |- | ||
| Line 52: | Line 41: | ||
|} | |} | ||
|} | |} | ||
Benzene, toluene, and xylenes can be made by various processes. However, most BTX production is based on the recovery of aromatics derived from the catalytic reforming of naphtha in a petroleum refinery. | Benzene, toluene, and xylenes can be made by various processes. However, most BTX production is based on the recovery of aromatics derived from the catalytic reforming of naphtha in a petroleum refinery.<ref name=Chem-Systems/><ref name=IEA-Book>International Energy Agency (2006).[http://books.google.com/books?id=s0spIzYtrCAC&pg=PA414&lpg=PA414&dq=Benzene+toluene+xylene+production&source=bl&ots=ttMkz7Hl5x&sig=_01oioDJ3HXTVmXXnpA8bPdzf94&hl=en&sa=X&ei=wYFjT4uVIJCFsALapc2cCw&ved=0CIUBEOgBMAw4UA#v=onepage&q=Benzene%20toluene%20xylene%20production&f=false <i>Energy Technology Perspectives</i>]. 1st Edition. Organisation for Economic Co-operation and Development (OECD). Page 414. ISBN 08070-1556-3.</ref> | ||
Catalytic reforming usually utilizes a feedstock naphtha that contains non-aromatic hydrocarbons with 6 to 11 or 12 carbon atoms and typically produces a '''''reformate''''' product containing C<sub>6</sub> to C<sub>8</sub> aromatics (benzene, toluene, xylenes) as well as paraffins and heavier aromatics containing 9 to 11 or 12 carbon atoms. | Catalytic reforming usually utilizes a feedstock naphtha that contains non-aromatic hydrocarbons with 6 to 11 or 12 carbon atoms and typically produces a '''''reformate''''' product containing C<sub>6</sub> to C<sub>8</sub> aromatics (benzene, toluene, xylenes) as well as paraffins and heavier aromatics containing 9 to 11 or 12 carbon atoms. | ||
Another process for producing BTX aromatics involves the steam cracking of hydrocarbons which typically produces a cracked naphtha product commonly referred to as '''''pyrolysis gasoline''''', '''''pyrolysis gas''''' or '''''pygas'''''. The pyrolysis gasoline typically consists of C<sub>6</sub> to C<sub>8</sub> aromatics, heavier aromatics containing 9 to 11 or 12 carbon atoms, and non-aromatic cyclic hydrocarbons (naphthenes) containing 6 or more carbon atoms. | Another process for producing BTX aromatics involves the steam cracking of hydrocarbons which typically produces a cracked naphtha product commonly referred to as '''''pyrolysis gasoline''''', '''''pyrolysis gas''''' or '''''pygas'''''. The pyrolysis gasoline typically consists of C<sub>6</sub> to C<sub>8</sub> aromatics, heavier aromatics containing 9 to 11 or 12 carbon atoms, and non-aromatic cyclic hydrocarbons (naphthenes) containing 6 or more carbon atoms.<ref name=Chem-Systems/><ref name=IEA-Book/> | ||
The adjacent table compares the BTX content of pyrolysis gasoline produced at standard cracking severity or at medium cracking severity with the BTX content of catalytic reformate produced by a either a continuous catalytic regenerative (CCR) reformer or by a semi-regenerative catalytic reformer. | The adjacent table compares the BTX content of pyrolysis gasoline produced at standard cracking severity or at medium cracking severity with the BTX content of catalytic reformate produced by a either a continuous catalytic regenerative (CCR) reformer or by a semi-regenerative catalytic reformer. | ||
| Line 68: | Line 57: | ||
==Petrochemicals produced from BTX== | ==Petrochemicals produced from BTX== | ||
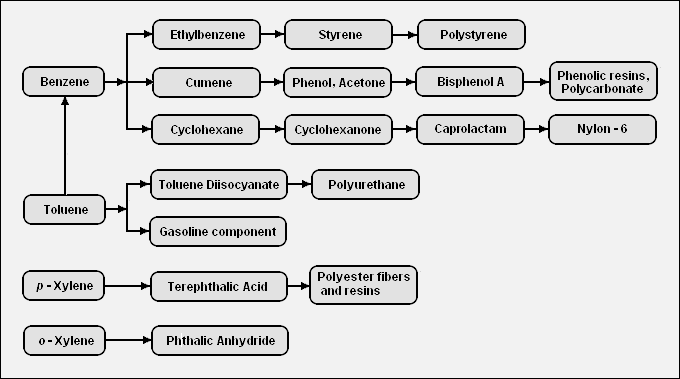
There are a very large number of petrochemicals produced from the BTX aromatics. The following diagram shows the chains leading from the BTX components to some of the petrochemicals that can be produced from those components: | There are a very large number of petrochemicals produced from the BTX aromatics. The following diagram shows the chains leading from the BTX components to some of the petrochemicals that can be produced from those components:<ref name=BTX-Chain/> | ||
<span style="clear:right;">[[Image:BTX Derivatives.png|680px|Diagram of the chain of petrochemicals derived from the BTX aromatics.]]</span> | <span style="clear:right;">[[Image:BTX Derivatives.png|680px|Diagram of the chain of petrochemicals derived from the BTX aromatics.]]</span> | ||
Revision as of 20:57, 17 March 2012
In the petroleum and petrochemical industries, the acronym BTX refers to mixtures of the aromatic hydrocarbons benzene, toluene, and the three xylene isomers. The xylene isomers are distinguished by the designations ortho – or o –, meta – or m –, and para – or p – as indicated in the adjacent diagram. If ethylbenzene is included, the mixture is sometimes referred to as BTEX.
The BTX aromatics are very important petrochemical materials. The world wide demand for these hydrocarbons is steadily increasing. The demand for xylenes, particularly para – xylene, has increased in proportion to the increase in demand for polyester fibers and film. Benzene is a highly valuable product for use as a chemical raw material. Toluene is also a valuable petrochemical for use as a solvent and intermediate in chemical manufacturing processes and as a high octane gasoline component.[1][2][3][4]
Properties of the BTX hydrocarbons
The table below lists some of the properties of the BTX aromatic hydrocarbons, all of which are liquids at typical room conditions:
| benzene | toluene | ethylbenzene | p - xylene | m - xylene | o - xylene | |
|---|---|---|---|---|---|---|
| Molecular formula | C6H6 | C7H8 | C8H10 | C8H10 | C8H10 | C8H10 |
| Molecular mass, g · mol –1 | 78.12 | 92.15 | 106.17 | 106.17 | 106.17 | 106.17 |
| Boiling point, °C | 80.1 | 110.6 | 136.2 | 138.4 | 139.1 | 144.4 |
| Melting point, °C | 5.5 | – 95.0 | – 95.0 | 13.3 | – 47.9 | – 25.2 |
Production of BTX hydrocarbons
| ||||||||||||||||||||||||||||||||||
Benzene, toluene, and xylenes can be made by various processes. However, most BTX production is based on the recovery of aromatics derived from the catalytic reforming of naphtha in a petroleum refinery.[3][6]
Catalytic reforming usually utilizes a feedstock naphtha that contains non-aromatic hydrocarbons with 6 to 11 or 12 carbon atoms and typically produces a reformate product containing C6 to C8 aromatics (benzene, toluene, xylenes) as well as paraffins and heavier aromatics containing 9 to 11 or 12 carbon atoms.
Another process for producing BTX aromatics involves the steam cracking of hydrocarbons which typically produces a cracked naphtha product commonly referred to as pyrolysis gasoline, pyrolysis gas or pygas. The pyrolysis gasoline typically consists of C6 to C8 aromatics, heavier aromatics containing 9 to 11 or 12 carbon atoms, and non-aromatic cyclic hydrocarbons (naphthenes) containing 6 or more carbon atoms.[3][6]
The adjacent table compares the BTX content of pyrolysis gasoline produced at standard cracking severity or at medium cracking severity with the BTX content of catalytic reformate produced by a either a continuous catalytic regenerative (CCR) reformer or by a semi-regenerative catalytic reformer.
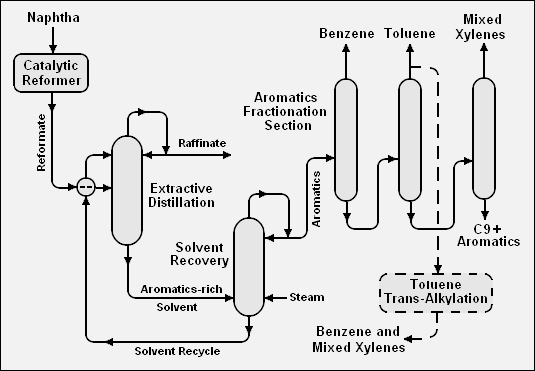
The BTX aromatics can be extracted from catalytic reformate or from pyrolysis gasoline by many different methods. Most of those methods, but not all, involve the use of a solvent either for liquid-liquid extraction or extractive distillation. Many different solvents are suitable, including sulfolane (C4H8O2S), furfural (C5H4O2), tetraethylene glycol (C8H18O5), dimethylsulfoxide (C2H6OS), and N-methyl-2-pyrrolidone (C5H9NO).
Below is a schematic flow diagram of one method, involving extractive distillation, for extraction of the BTX aromatics from a catalytic reformate:
Petrochemicals produced from BTX
There are a very large number of petrochemicals produced from the BTX aromatics. The following diagram shows the chains leading from the BTX components to some of the petrochemicals that can be produced from those components:[1]
References
- ↑ 1.0 1.1 The BTX Chain: Benzene, Toluene, Xylene. Chapter 4 of the DOE's Office of Energy Efficiency and Renewable Energy (EERE) report entitled "Energy and Environmental Profile of the U.S. Chemical Industry" of May 2000.
- ↑ Use of Process Analytics in Aromatics (BTX and phenol) production plants. Case Study, August 2008. (scroll down to "Aromatics" section.
- ↑ 3.0 3.1 3.2 3.3 Benzene/Toluene. Introduction to a ChemSystems report, 2009.
- ↑ 10.6 Aromatics, Online Italian Encyclopedia of Hydrocarbons, Istituto della Enciclopedia Italiana, Volume II, 2006, pages 603-605.
- ↑ Robert C. Weast (Editor) (1975). Handbook of Chemistry and Physics, 56th Edition. CRC Press. ISBN 0-87819-455-X.
- ↑ 6.0 6.1 International Energy Agency (2006).Energy Technology Perspectives. 1st Edition. Organisation for Economic Co-operation and Development (OECD). Page 414. ISBN 08070-1556-3.