User:Milton Beychok/Sandbox: Difference between revisions
imported>Milton Beychok No edit summary |
imported>Milton Beychok No edit summary |
||
| Line 14: | Line 14: | ||
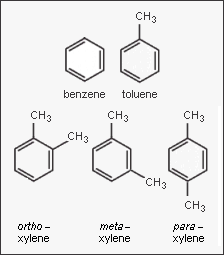
{{Image|BTX.png|right|350px|Structural diagrams of the BTX aromatic hydrocarbons.}} | {{Image|BTX.png|right|350px|Structural diagrams of the BTX aromatic hydrocarbons.}} | ||
In the petroleum and petrochemical industries, the acronym '''BTX''' refers to mixtures of the aromatic hydrocarbons benzene, toluene, and the three xylene isomers. If ethylbenzene is included, the mixture is sometimes referred to '''BTEX'''. The xylene isomers are distinguished by the designations ''ortho'' – | In the petroleum and petrochemical industries, the acronym '''BTX''' refers to mixtures of the aromatic hydrocarbons benzene, toluene, and the three xylene isomers. If ethylbenzene is included, the mixture is sometimes referred to '''BTEX'''. The xylene isomers are distinguished by the designations ''ortho'' – or ''o'' –, ''meta'' – or ''m'' –, and ''para'' – or ''p'' – as indicated in the adjacent diagram. | ||
The BTX aromatics are very important petrochemical materials. The world wide demand for these hydrocarbons is steadily increasing. The demand for xylenes, particularly ''para''  | The BTX aromatics are very important petrochemical materials. The world wide demand for these hydrocarbons is steadily increasing. The demand for xylenes, particularly ''para'' – xylene, has increased in proportion to the increase in demand for polyester fibers and film. Benzene is a highly valuable product for use as a chemical raw material. Toluene is also a valuable petrochemical for use as a solvent and intermediate in chemical manufacturing processes and as a high octane gasoline component. | ||
==Properties of the BTX hydrocarbons== | |||
The table below lists some of the properties of the BTX aromatic hydrocarbons, all of which are liquids at typical room conditions: | |||
{|class=wikitable align= | {|class=wikitable align= | ||
|+Properties(ref name=CRC-Hdbk/) | |+Properties(ref name=CRC-Hdbk/) | ||
| Line 36: | Line 33: | ||
|Melting point, °C||5.5||– 95.0||– 95.0||13.3||– 47.9||– 25.2 | |Melting point, °C||5.5||– 95.0||– 95.0||13.3||– 47.9||– 25.2 | ||
|} | |} | ||
==Production of BTX hydrocarbons== | |||
Benzene, toluene, and xylenes can be made by various processes. Most BTX production is based on the recovery of aromatics derived from the catalytic reforming of naphtha in a petroleum refinery. Catalytic reforming utilizes a feed containing C6+ non-aromatic hydrocarbons and typically produces a "reformate" liquid containing of C<sub>6</sub> to C<sub>8</sub> aromatics (benzene, toluene, xylenes) as well as paraffins and heavier aromatics. | |||
Another process for producing BTX aromatics involves the steam cracking of hydrocarbons which typically produces a cracked naphtha product consisting of C6+ non-aromatic cyclic hydrocarbons, A6-A8 aromatic hydrocarbons (benzene, toluene, xylenes and ethylbenzene), and A9+ aromatic hydrocarbons. | |||
Revision as of 12:21, 17 March 2012
References
- ↑ The BTX Chain: Benzene, Toluene, Xylene. Chapter 4 of the DOE's Office of Energy Efficiency and Renewable Energy (EERE) report entitled "Energy and Environmental Profile of the U.S. Chemical Industry" of May 2000.
- ↑ Benzene/Toluene. Introduction to a ChemSystems report, 2009.
- ↑ Use of Process Analytics in Aromatics (BTX and phenol) production plants. Case Study, August 2008.
- ↑ International Energy Agency (2006).Energy Technology Perspectives. 1st Edition. Organisation for Economic Co-operation and Development (OECD). Page 414. ISBN 08070-1556-3.
- ↑ 10.6 Aromatics, Online Italian Encyclopedia of Hydrocarbons, Istituto della Enciclopedia Italiana, Volume II, 2006, pages 603-605.
- ↑ Robert C. Weast (Editor) (1975). Handbook of Chemistry and Physics, 56th Edition. CRC Press. ISBN 0-87819-455-X.
In the petroleum and petrochemical industries, the acronym BTX refers to mixtures of the aromatic hydrocarbons benzene, toluene, and the three xylene isomers. If ethylbenzene is included, the mixture is sometimes referred to BTEX. The xylene isomers are distinguished by the designations ortho – or o –, meta – or m –, and para – or p – as indicated in the adjacent diagram.
The BTX aromatics are very important petrochemical materials. The world wide demand for these hydrocarbons is steadily increasing. The demand for xylenes, particularly para – xylene, has increased in proportion to the increase in demand for polyester fibers and film. Benzene is a highly valuable product for use as a chemical raw material. Toluene is also a valuable petrochemical for use as a solvent and intermediate in chemical manufacturing processes and as a high octane gasoline component.
Properties of the BTX hydrocarbons
The table below lists some of the properties of the BTX aromatic hydrocarbons, all of which are liquids at typical room conditions:
| benzene | toluene | ethylbenzene | p - xylene | m - xylene | o - xylene | |
|---|---|---|---|---|---|---|
| Molecular formula | C6H6 | C7H8 | C8H10 | C8H10 | C8H10 | C8H10 |
| Molecular mass, g · mol –1 | 78.12 | 92.15 | 106.17 | 106.17 | 106.17 | 106.17 |
| Boiling point, °C | 80.1 | 110.6 | 136.2 | 138.4 | 139.1 | 144.4 |
| Melting point, °C | 5.5 | – 95.0 | – 95.0 | 13.3 | – 47.9 | – 25.2 |
Production of BTX hydrocarbons
Benzene, toluene, and xylenes can be made by various processes. Most BTX production is based on the recovery of aromatics derived from the catalytic reforming of naphtha in a petroleum refinery. Catalytic reforming utilizes a feed containing C6+ non-aromatic hydrocarbons and typically produces a "reformate" liquid containing of C6 to C8 aromatics (benzene, toluene, xylenes) as well as paraffins and heavier aromatics.
Another process for producing BTX aromatics involves the steam cracking of hydrocarbons which typically produces a cracked naphtha product consisting of C6+ non-aromatic cyclic hydrocarbons, A6-A8 aromatic hydrocarbons (benzene, toluene, xylenes and ethylbenzene), and A9+ aromatic hydrocarbons.