Pregabalin: Difference between revisions
imported>Robert Badgett (Started Adverse effects) |
imported>Robert Badgett m (fixed dailymed citations) |
||
| Line 2: | Line 2: | ||
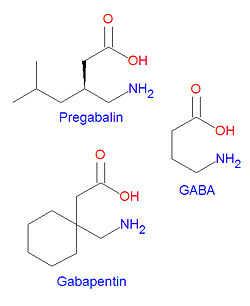
{{Image|Pregabalin and related compounds.jpg|right|250px|Pregabalin and gabapentin are structurally similar to GABA.}} | {{Image|Pregabalin and related compounds.jpg|right|250px|Pregabalin and gabapentin are structurally similar to GABA.}} | ||
'''Pregabalin''' is structurally similar to [[gabapentin]]. It is also similar to the inhibitory neurotransmitter gamma-aminobutyric acid ([[GABA]]), and although it does not act on GABA receptors, it may increase the "density of GABA transporter protein and increases the rate of functional GABA transport".<ref name="DailyMed">{{ | '''Pregabalin''', Lyrica®, is structurally similar to [[gabapentin]]. It is also similar to the inhibitory neurotransmitter gamma-aminobutyric acid ([[GABA]]), and although it does not act on GABA receptors, it may increase the "density of GABA transporter protein and increases the rate of functional GABA transport".<ref name="DailyMed">{{DailyMed}} | ||
</ref> It is approved by the FDA for neuropathic pain associated with [[diabetic neuropathy|diabetic peripheral neuropathy]], postherpetic neuralgia adjunctive therapy for adult patients with partial onset [[seizure]]s, and [[fibromyalgia]].<ref name="DailyMed">{{ | </ref> It is approved by the FDA for neuropathic pain associated with [[diabetic neuropathy|diabetic peripheral neuropathy]], postherpetic neuralgia adjunctive therapy for adult patients with partial onset [[seizure]]s, and [[fibromyalgia]].<ref name="DailyMed">{{DailyMed}} | ||
</ref> | </ref> | ||
| Line 16: | Line 16: | ||
== External links == | == External links == | ||
{{CZMed}} | {{CZMed}} | ||
==References== | ==References== | ||
<references/> | <references/> | ||
Revision as of 09:21, 19 March 2010
Pregabalin, Lyrica®, is structurally similar to gabapentin. It is also similar to the inhibitory neurotransmitter gamma-aminobutyric acid (GABA), and although it does not act on GABA receptors, it may increase the "density of GABA transporter protein and increases the rate of functional GABA transport".[1] It is approved by the FDA for neuropathic pain associated with diabetic peripheral neuropathy, postherpetic neuralgia adjunctive therapy for adult patients with partial onset seizures, and fibromyalgia.[1]
Chemistry
The IUPAC chemical name for pregabalin is (S)-(+)-3-(aminomethyl)-5-methylhexanoic acid. Its chemical formula is C8H17NO2 giving it a molecular mass of 159.23 g/mol. It is both an amine and a carboxylic acid.
Drug toxicity
At maximum doses of 600 mg per day, drug toxicity from pregabalin may include reduced cognitive function.[2]
External links
The most up-to-date information about Pregabalin and other drugs can be found at the following sites.
- Pregabalin - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Pregabalin - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Pregabalin - Detailed information from DrugBank.
References
- ↑ 1.0 1.1 Pregabalin - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- ↑ Salinsky M, Storzbach D, Munoz S (2010). "Cognitive effects of pregabalin in healthy volunteers: a double-blind, placebo-controlled trial.". Neurology 74 (9): 755-61. DOI:10.1212/WNL.0b013e3181d25b34. PMID 20194915. Research Blogging.