Brucella canis: Difference between revisions
imported>Aleisha Murdock |
imported>Aleisha Murdock |
||
| Line 9: | Line 9: | ||
Brucella canis are gram negative | Brucella canis are gram-negative Proteobacteria within the Brucellacae family. B. canis is a facultative intracellular pathogen that can survive well outside a host, but prefer to reproduce within the host. When first found B. canis was one of many species of Brucella, but in 1977 it was determined that the only species is Brucella melitensis and the others are just at subtypes of the species. The subtypes only exist for their differences in the animal host it prefers and phenotypic appearance. B. canis is one of the subtypes with the preference for dogs as its host (“Brucella,” 2009). | ||
Revision as of 14:25, 9 May 2009
For the course duration, the article is closed to outside editing. Of course you can always leave comments on the discussion page. The anticipated date of course completion is May 21, 2009. One month after that date at the latest, this notice shall be removed. Besides, many other Citizendium articles welcome your collaboration! |
Description and Significance
Brucella canis are gram-negative Proteobacteria within the Brucellacae family. B. canis is a facultative intracellular pathogen that can survive well outside a host, but prefer to reproduce within the host. When first found B. canis was one of many species of Brucella, but in 1977 it was determined that the only species is Brucella melitensis and the others are just at subtypes of the species. The subtypes only exist for their differences in the animal host it prefers and phenotypic appearance. B. canis is one of the subtypes with the preference for dogs as its host (“Brucella,” 2009).
Genome
The genome of Brucella canis consists of two circular chromosomes structured as one large chromosome and a smaller chromosome. Chromosome I consists of 2,105,969 and 2,153 genes 2,153 while chromosome II consists of 1,206,800 bp and 1,671 genes. On both chromosomes there are genes that code for DNA replication, protein synthesis, core metabolism, and cell-wall biosynthesis ( DelVecchio et al.).
Cell Structure and Metabolism
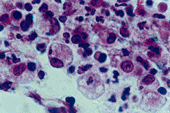
Brucella canis are small, rough, rod shaped, and non motile gram negative bacteria. Often times it is termed coccobacillai shaped because it looks like short rods rather than long rods. (“Brucellosis” 1999). The significance of the structure of the bacterium is that it has the ability to act as a pathogen, but lacks the structural characteristics that most pathogenic viruses have. B. canis growth is inhibited by a 10% concentration of carbon dioxide (“Brucella”).
Ecology
Brucella canis are found all over the world, but more specifically areas that have poor sanitation and lack good public health safety to support animal infection or lack economic funds to support public health. Some areas where B. canis have been reported are the southern states within the United States, Canada, South America with Mexico included, Central America, Europe, Tunisia, Nigeria, Madagascar, Malaysia, India, Korea, Japan, and China. Australia and New Zealand may be the only areas of the world that do not contain the bacterium (“Canine Brucellosis: Brucella canis”). In Arizona pet diseases are not reported to the department of health making it unknown if the bacterium exists in that area. B. canis can withstand various environmental changes ranged from very high temperatures, lack of sunlight, water, feces, urine, and clothing for several months with out need of an intracellular host (“Brucella canis Q & A for Veterinarians”).
Pathology
Transmission
B. canis can penetrate mucous membranes within the oral cavity and vagina of dogs. The bacterium is found living within the uterine tissue and secretions of female dogs and is shed for months after an abortion. The bacteria found in the semen, testicles, and urine of male dogs and are shed the bacteria for years even past death. B. canis can live within a dog for years before it gets rid of the infection. B. cains is transmitted from dog to dog sexually through vaginal and semen secretions. The bacteria can release from infected dogs by secretions that exit the mouth and nose, and through scars or cuts. Although, dogs are the common host B. canis is zoonotic enabling an infected dogs can cause spread to humans by direct contact with humans usually by just touching an infected dog. The bacterium is mostly exposed to humans that work with the infected animals or the bacterium such as vets and lab workers (Canine Brucellosis: Brucella canis”)
Symptoms
The most common symptoms in female dogs infected by Brucella canis is infertility where abortion occurs during the 45-59 days of pregnancy (“Canine Brucellosis”). Puppies that are born die 2-3 weeks after birth and those that survive carry B. canis in their blood for months (“Canine Brucellosis: Brucella canis”). In males dogs the most common symptom is infertility because of abnormal sperm count, and tesitile atrophy. The most common symptoms in humans are flu like with fever, headache, seating, body aches, and weight loss. It takes 8 days after onset to diagnosis the disease.
Diagnosis
Brucella canis can be diagnosed in an infected dog by using serologic tests and blood cultures. Although useful, serological tests are not the best way to diagnosis B. canis because the antigens they test for cross-react with antibodies of gram negative bacteria that are not pathogenic rather then just the antibodies formed in response to the infection. Blood cultures can be used to confirm a positive serologic test and is more convincing because the presence of B. canis indicates an infection has occurred. The bacterium can be found in the blood of an infected dog 2-4 weeks after infection and survives for 5 or more years (“Brucella canis Q & A for Veterinarians”).
Treatment
Once a dog is infected by Brucella canis it is difficult for the dog to become fully cured. Finding an effective treatment for brucellosis has been very difficult because the bacterium is embedded within the mucous cells of the animal. Antibiotics have been found to have some effect on dogs infected with the bacteria, but only by using many different antibiotics. The problem with antibiotics is that the dog may relapse and the bacteria count is only reduced and not completely removed regardless of the use of the antibiotics. A method of spraying has been used on dogs to reduce spread of the bacterium to others, but unfortunately like the antibiotics it does not remove the bacterium. Long after the bacterium has been successfully treated the dog may still release the bacterium making it difficult for them to try to reproduce after it is claimed to be cured (“Brucella canis Q & A for Veterinarians”). Antibiotics can also be used to cure infected humans and humans respond more quickly to antibiotics (Shin, S., and L. E. Carmichael).
Prevention
Brucella canis is more of a sicking than it is killing. The mortality rate of B. canis is very low and deaths only occur if a person gets brucella endocarditis or meningitis (“Canine Brucellosis: Brucella canis”). Unfortunately there is still no vaccine to prevent brucellosis. All breeding dogs are tested year round to prevent the spread of Brucella canis. Infected dogs are isolated immediately from other dogs and not allowed to breed even after it may be cured. Humans can protect themselves by using gloves and cleaning by using disinfectants when handling B. canis or infected dogs (“Brucella canis Q & A for Veterinarians”).
Current Research
Genome
A whole genome based phylogeny has been created from 13 genomes of Brucella species using the closely related bacterium Ochrobacterium (Foster et al.). The purpose was to determine if the phylogeny design reflects the phylogeny of the preferred host. On each chromosome of the Brucella species there are SARs or shared anonalongs regions that are not found on Ochrobacterium bringing fourth the fact that chromosome structure are independent of its intracellular life cycle. The SARs contain many genes that the bacterium needs to survive and if mutations that occur on any the SARs genes the ability for the Brucella to survive within its host is reduced (Wattam et al.).
Human infection
In order to do serologic test of Brucella canis a less muciod of M- strain of B. canis is used rather than the wild type because the wild type aggregate when the specific antibodies are absent. When M- strain of is injected into infected dogs the strain reduced virulence of the bacterium. This led researchers to wonder if the M- strain is avirulent in humans as well. According to a case reported by the CDC, a 35 year old male was working with B. canis M- strain in a lab without using any safety precautions. The man was not wearing any gloves and used his mouth to handle the pipette. The man did not have any contact with any dogs, but was showing symptoms of brucellosis and when tested he was found positive for B. canis. The man had been infected by the M- strain. The man went through 4 years of antibiotic therapy before he was cured. The man still works with B. canis M- strain, but uses googles, gloves, masks, and protects himself from being contaminated. It is shown that M- strain is helpful in infected dogs, but not helpful in humans because it causes the same symptoms as wild type B. canis (Wallach et al.).
Brucellosis Endocarditis
Although Brucella canis is the significant cause of brucellosis in dogs it is rare in humans. According to a case report a 49 year old man was hospitalized because he was experiencing fever, weight loss, and heart murmur. After a serologic test was performed it showed that B. canis was the causal agent of the heart complications. The man stated that he had not been in contact with any dogs or labs thereby making the source of the infection to have come from something else. The man was cured heart valve removal and after 3 years of antibiotic therapy. This report is still undergoing further analysis because of hoe rare it is for humans to get brucella endocarditis (Ying et al.).
Reference
Brucella. 2009. 21, Apr. 2009 <http://medical-dictionary.thefreedictionary.com/Brucella+canis>.
Brucella canis Q & A for Veterinarians. 21, Apr. 2009 <http://www.azdhs.gov/phs/oids/vector/brucella/pdf/bruc_qa_vets.pdf>.
Brucellosis. 2009. 21, Apr. 2009 <http://www.vetinfo.com/dencyclopedia/debrucel.html>.
Brucellosis. 1999. 21, Apr. 2009 <http://www.cbwinfo.com/Biological/Pathogens/BM.shtml>.
Canine Brucellosis. 2000. 21, Apr. 2009 <http://images.google.com/imgres? imgurl=http://publications.royalcanin.com/images/2/2472/p256.jpg&imgrefurl>.
Centers for Disease Control and Prevention. Brucellosis. 2007. 21, Apr. 2009 <http://www.cdc.gov/ncidod/dbmd/diseaseinfo/Brucellosis_g.htm>.
DelVecchio, Vito G., et al. “The genome sequence of the facultative intracellular pathogen Brucella melitensis” 99 (2002): 443-448.
Drs. Foster & Smith, Inc. Brucellosis (Brucella canis) & Abortions in Dogs. 1997. 21 Apr. 2009 <http://www.peteducation.com/article>
Foster, JT., et al.“Whole genome-based phylogeny and divergence of the genus Brucella” J Bacteriol (2009).
Lucero, Nidia E., et al. “Diagnosis of human brucellosis caused by Brucella canis” Journal of Medical Microbiology 54 (2005): 457-461.
Shin, S., and L. E. Carmichael. “Canine Brucellosis caused by Brucella canis”. 1999.
The Center for Food Security and Public Health. Canine Brucellosis: Brucella canis. 2007. 21, Apr. 2009 <http://www.cfsph.iastate.edu/Factsheets/pdfs/brucellosis_canis.pdf>.
Wallach, Jorge C., et al. “Human infection with M- strain of Brucella canis” Emerging Infectious Diseases 10 (2004).
Wattam, AR., et al. “Analysis of Brucella genomes reveals evidence for horizontal gene transfer despite a preferred intracellular lifecycle” J Bacteriol (2009).
Wikimedia Foundation, Inc. Brucella canis. 2009. 21 Apr. 2009 <http://en.wikipedia.org/wiki/Brucella_abortus>.
Ying, Wang., Minh Q. Nguyen, and Jeffrey A. Jahre. “Brucella canis Endocarditis” Clinical Infectious Disease 29 (1999) 1593-1594.