Temazepam: Difference between revisions
imported>Francis Zaia (Created page with "{{Subpages}} {{Image|Temazepam.png|right|250px|}} '''Temazepam''' (marketed under brand names '''Restoril''', '''Euhypnos''', '''Normison''', '''Remestan''', '''Tenox''' and '''...") |
Pat Palmer (talk | contribs) m (Text replacement - "United States" to "United States") |
||
| Line 5: | Line 5: | ||
==History== | ==History== | ||
Temazepam was first synthesized in 1964 but did not come into use as an hypnotic until 1969.<ref>{{cite journal |author=Maggini C |coauthors=Murri M, Sacchetti G |journal=Arzneimittelforschung |year=1969 |month=October |title=Evaluation of the effectiveness of temazepam on the insomnia of patients with neurosis and endogenous depression |volume=19 |issue=10 |pages=1647–52 |pmid=4311716}}</ref> In the [[United States]] it was not approved for the treatment of insomnia by the [[Food and Drug Administration]] until 1981 when it came out under the brand name ''Restoril''. It quickly gained popularity as an effective sleeping pill but with the rise in prescriptions also came the rise in abuse of the drug and addiction became rampant, particularly in [[Europe]] and [[Australia]]. | Temazepam was first synthesized in 1964 but did not come into use as an hypnotic until 1969.<ref>{{cite journal |author=Maggini C |coauthors=Murri M, Sacchetti G |journal=Arzneimittelforschung |year=1969 |month=October |title=Evaluation of the effectiveness of temazepam on the insomnia of patients with neurosis and endogenous depression |volume=19 |issue=10 |pages=1647–52 |pmid=4311716}}</ref> In the [[United States of America|United States]] it was not approved for the treatment of insomnia by the [[Food and Drug Administration]] until 1981 when it came out under the brand name ''Restoril''. It quickly gained popularity as an effective sleeping pill but with the rise in prescriptions also came the rise in abuse of the drug and addiction became rampant, particularly in [[Europe]] and [[Australia]]. | ||
==Pharmacology== | ==Pharmacology== | ||
Revision as of 13:32, 2 February 2023
Temazepam (marketed under brand names Restoril, Euhypnos, Normison, Remestan, Tenox and Norkotral) is a powerful short to intermediate-acting sedative-hypnotic benzodiazepine drug of the 3-hydroxy category. Therapeutic and supratherapeutic doses of the drug can manifest clinical effects of strong hypnosis, sedation, amnesia, and ataxia. In addition, temazepam has effective anxiolytic (anti-anxiety), anticonvulsant, and skeletal muscle relaxant properties. After an oral dose of temazepam, absorption occurs rapidly to induce sleep and preserve sleep architecture. It is generally prescribed for the treatment of short-term severe or debilitating insomnia in patients who have difficulty falling asleep or maintaining sleep.[1][2][3]
History
Temazepam was first synthesized in 1964 but did not come into use as an hypnotic until 1969.[4] In the United States it was not approved for the treatment of insomnia by the Food and Drug Administration until 1981 when it came out under the brand name Restoril. It quickly gained popularity as an effective sleeping pill but with the rise in prescriptions also came the rise in abuse of the drug and addiction became rampant, particularly in Europe and Australia.
Pharmacology
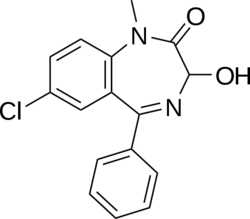
Temazepam is classed as a 1,4 benzodiazepine, with the chemical name 7-chloro-1,3-dihydro-3-hydroxy-1-methyl-5-phenyl-2H-1-4-benzodiazepin-2-one. It is a white, crystalline substance, is very slightly soluble in water and sparingly soluble in alcohol. The main pharmacological action of temazepam is the enhancement of the neurotransmitter, GABA at the GABAA receptor. Modulation of the α1 is associated with sedation, motor-impairment, anxiolysis, amnesia, muscle relaxation, anticonvulsant effects, ataxia, and reinforcing behavior.[5] It is a full agonist of the benzodiazepine receptor and its half-life is 8-22 hours.
Pharmacokinetics
Oral administration of 15 to 45 mg temazepam in man resulted in rapid absorption with significant blood levels achieved in less than 30 minutes and peak levels at 2 to 3 hours. In a single and multiple dose absorption, distribution, metabolism, and excretion (ADME) study, using tritium (3H) labelled drug, temazepam was well absorbed and found to have minimal (8%) first pass metabolism. There were no active metabolites formed and the only significant metabolite present in blood was the O-conjugate. The unchanged drug was 96% bound to plasma proteins. The blood level decline of the parent drug was biphasic with the short half-life ranging from 0.4-0.6 hours and the terminal half-life from 3.5-18.4 hours (mean 8.8 hours), depending on the study population and method of determination.[6] Metabolites were formed with a half-life of 10 hours and excreted with a half-life of approximately 2 hours. Thus, formation of the major metabolite is the rate limiting step in the biodisposition of temazepam. There is no accumulation of metabolites. A dose-proportional relationship has been established for the area under the plasma concentration/time curve over the 15-30 mg dose range.
Temazepam was completely metabolized through conjugation prior to excretion; 80%-90% of the dose appeared in the urine. The major metabolite was the O-conjugate of temazepam (90%); the O-conjugate of N-desmethyl temazepam was a minor metabolite (7%).[7]
Bioavailability, Induction, and Plasma Levels
After oral administration, temazepam is rapidly absorbed and fast acting. Following ingestion of 30 mg temazepam, measurable plasma concentrations are achieved 10-20 minutes after dosing with peak plasma levels ranging from 666-982 ng/mL (mean 865 ng/mL) occurring approximately 1.2-1.6 hours (mean 1.5 hours) after dosing. Strong hypnotic, sedative and anticonvulsant effects are noticeable 20-30 minutes after ingestion.
In a 7 day study, in which subjects were given a 30 mg temazepam capsule 1 hour before retiring, steady-state (as measured by the attainment of maximal trough concentrations) was achieved by the third dose. Mean plasma levels of temazepam (for days 2-7) were 260±210 ng/mL at 9 hours and 75±80 ng/mL at 24 hours after dosing. A slight trend toward declining 24 hour plasma levels was seen after day 4 in the study, however, the 24 hour plasma levels were quite variable. At a dose of 30 mg once-a-day for 8 weeks, no evidence of enzyme induction was found in man.
Cited references
- ↑ Wynn RL; McFarland SA, Meyer EA, Coffman WB, Mumford R (1980). "Structure activity relationships of selected benzodiazepines as anticonvulsants to local anesthetics". Pharmacol Ther Dent 5 (1-2): 39–45. PMID 6774348.
- ↑ Siciliani O; Schiavon M, Tansella M (August 1975). "Anxiety and EEG alpha activity in neurotic patients". Acta Psychiatr Scand 52 (2): 116–31. DOI:10.1111/j.1600-0447.1975.tb00028.x. PMID 1096540. Research Blogging.
- ↑ Fuccella LM (1979). "Bioavailability of temazepam in soft gelatin capsules" (PDF). Br J Clin Pharmacol 8 (1): 31S–5S. PMID 41540. PMC 1429631.
- ↑ Maggini C; Murri M, Sacchetti G (October 1969). "Evaluation of the effectiveness of temazepam on the insomnia of patients with neurosis and endogenous depression". Arzneimittelforschung 19 (10): 1647–52. PMID 4311716.
- ↑ Oelschläger H (July 4, 1989). "[Chemical and pharmacologic aspects of benzodiazepines]". Schweiz Rundsch Med Prax 78 (27–28): 766–72. PMID 2570451.
- ↑ Müller FO, Van Dyk M, Hundt HK, et al. (1987). "Pharmacokinetics of temazepam after day-time and night-time oral administration". Eur. J. Clin. Pharmacol 33 (2): 211–4. DOI:10.1007/BF00544571. PMID 2891534. Research Blogging.
- ↑ Schwarz HJ (August 1, 1979). "Pharmacokinetics and metabolism of temazepam in man and several animal species" (PDF). British Journal of Clinical Pharmacology 8 (1): 23S–29S. PMID 41539. PMC 1429628.