Asparagine: Difference between revisions
Jump to navigation
Jump to search

imported>David E. Volk No edit summary |
imported>Caesar Schinas m (Bot: Update image code) |
||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
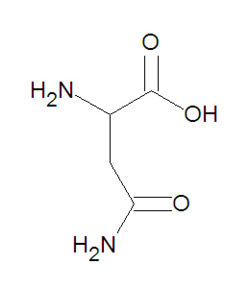
{{Image|Asparagine DEVolk.jpg|right|250px|Asparagine (ASP, D), a common amino acid.}} | |||
'''Asparagine''', abbreviated either as '''ASP''' or '''D''', is one of the twenty common [[amino acid]]s used by living organisms to build [[protein]]s. It is one of the neutral, polar amino acids. The ''side chain'' of asparagine is the amide group -CH<sub>2</sub>-C(O)-NH<sub>2</sub>. This side chain is capable of forming [[hydrogen bond]]s with other chemical entities that are electron donors. It is very similar to [[glutamine]], which has one extra carbon on the side chain before the amide group, and [[aspartic acid]], which has a [[hydroxyl]] group (OH) in place of the NH<sub>2</sub> moiety on the side chain. | '''Asparagine''', abbreviated either as '''ASP''' or '''D''', is one of the twenty common [[amino acid]]s used by living organisms to build [[protein]]s. It is one of the neutral, polar amino acids. The ''side chain'' of asparagine is the amide group -CH<sub>2</sub>-C(O)-NH<sub>2</sub>. This side chain is capable of forming [[hydrogen bond]]s with other chemical entities that are electron donors. It is very similar to [[glutamine]], which has one extra carbon on the side chain before the amide group, and [[aspartic acid]], which has a [[hydroxyl]] group (OH) in place of the NH<sub>2</sub> moiety on the side chain. | ||
Revision as of 05:17, 8 June 2009
Asparagine, abbreviated either as ASP or D, is one of the twenty common amino acids used by living organisms to build proteins. It is one of the neutral, polar amino acids. The side chain of asparagine is the amide group -CH2-C(O)-NH2. This side chain is capable of forming hydrogen bonds with other chemical entities that are electron donors. It is very similar to glutamine, which has one extra carbon on the side chain before the amide group, and aspartic acid, which has a hydroxyl group (OH) in place of the NH2 moiety on the side chain.