User:Milton Beychok/Sandbox: Difference between revisions
imported>Milton Beychok No edit summary |
imported>Milton Beychok No edit summary |
||
| Line 1: | Line 1: | ||
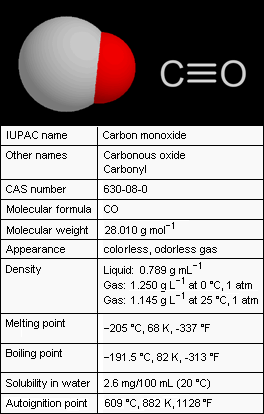
'''Carbon monoxide''' (CO), also referred to as '''carbonous oxide''', is a colorless, odorless, and tasteless [[gas]] that is slightly lighter than air. Exposure to high levels of carbon monoxide is extremely toxic to humans and animals. Conversely, small amounts of carbon monoxide are produced in normal animal metabolism and it is thought to have some normal biological functions. | {{Image|CarbonMonoxideProps.png|right|264px|Add image caption here.}} '''Carbon monoxide''' (CO), also referred to as '''carbonous oxide''', is a colorless, odorless, and tasteless [[gas]] that is slightly lighter than air. Exposure to high levels of carbon monoxide is extremely toxic to humans and animals. Conversely, small amounts of carbon monoxide are produced in normal animal metabolism and it is thought to have some normal biological functions. | ||
Carbon monoxide consists of one [[carbon]] [[atom]] and one [[oxygen]] atom. It is the simplest member of the class of inorganic compounds known as [[oxocarbons]] which includes [[carbon dioxide]] (CO<sub>2</sub>), [[carbon suboxide]] (C<sub>3</sub>O<sub>2</sub>), [[mellitic anhydride]] (C<sub>12</sub>O<sub>9</sub>) and many others. When combined with a metal (i.e., an [[organometallic]] complex), the carbon monoxide is a [[ligand]] called ''carbonyl'' : for example, in nickel carbonyl with the formula Ni(CO)<sub>4</sub>. | Carbon monoxide consists of one [[carbon]] [[atom]] and one [[oxygen]] atom. It is the simplest member of the class of inorganic compounds known as [[oxocarbons]] which includes [[carbon dioxide]] (CO<sub>2</sub>), [[carbon suboxide]] (C<sub>3</sub>O<sub>2</sub>), [[mellitic anhydride]] (C<sub>12</sub>O<sub>9</sub>) and many others. When combined with a metal (i.e., an [[organometallic]] complex), the carbon monoxide is a [[ligand]] called ''carbonyl'' : for example, in nickel carbonyl with the formula Ni(CO)<sub>4</sub>. | ||
Revision as of 14:57, 6 November 2011
Carbon monoxide (CO), also referred to as carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. Exposure to high levels of carbon monoxide is extremely toxic to humans and animals. Conversely, small amounts of carbon monoxide are produced in normal animal metabolism and it is thought to have some normal biological functions.
Carbon monoxide consists of one carbon atom and one oxygen atom. It is the simplest member of the class of inorganic compounds known as oxocarbons which includes carbon dioxide (CO2), carbon suboxide (C3O2), mellitic anhydride (C12O9) and many others. When combined with a metal (i.e., an organometallic complex), the carbon monoxide is a ligand called carbonyl : for example, in nickel carbonyl with the formula Ni(CO)4.
Carbon monoxide is produced by the partial combustion of carbon-containing substances. It is produced when there is not enough oxygen to form carbon dioxide, such as when operating a stove or an internal combustion engine in an enclosed space.
In the presence of oxygen, carbon monoxide burns with a blue flame, producing carbon dioxide.[1] Coal gas, which was widely used before the 1960s for domestic lighting, cooking, and heating, had carbon monoxide as a significant constituent. Iron smelting and other current technological processes still produce byproduct carbon monoxide.[2]
Worldwide, the largest source of carbon monoxide is from the photochemical reactions in the troposphere that generate about 5 x 1012 kilograms per year.[3] Other natural sources of CO include volcanoes, forest fires, and other forms of combustion.