User:Milton Beychok/Sandbox: Difference between revisions
imported>Milton Beychok No edit summary |
imported>Milton Beychok No edit summary |
||
| Line 1: | Line 1: | ||
{{TOC|right}} | |||
'''Liquefied natural gas''' or '''LNG''' is [[natural gas]] (predominantly [[methane]], CH<sub>4</sub>) that has been converted into liquid form for ease of transport and storage. | |||
A typical raw natural gas contains only about 80% methane and a number of higher boiling [[hydrocarbons]] as well as a number of impurities. Before it is liquefied, it is typically purified so as to remove the higher-boiling hydrocarbons and the impurities. The resultant liquefied natural gas contains about 95% or more methane and it is a | |||
clear, colorless and essentially odorless liquid which is neither corrosive nor toxic.<ref name=CalifEnergyCommission>[http://www.energy,ca.gov./faq.html Frequently Asked Questions About LNG] From the website of the [[California Energy Commission]]</ref> | |||
LNG occupies only a very small fraction (1/600th) of the volume of natural gas and is therefore more economical to transport across large distances. It can also be stored in large quantities that would be impractical for storage as a gas. | |||
==Process description for the production of LNG== | |||
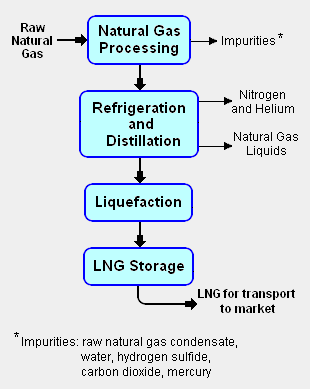
{{Image|LNG block flow diagram.png|right|310px|Add image caption here.}} | {{Image|LNG block flow diagram.png|right|310px|Add image caption here.}} | ||
The | The liquefaction process involves separating the raw natural gas from any associated water and high-boiling hydrocarbon liquids (referred to as [[natural gas condensate]]) that may be associated with the raw gas. The raw gas is then further purified in a [[natural gas processing]] plant to remove remove impurities such as the [[acid gas]]es hydrogen sulfide (H<sub>2</sub>S) and carbon dioxide (CO<sub>2</sub>), any residual water liquid or vapor, [[mercury]], [[nitrogen]] and [[helium]] which could cause difficulty downstream. | ||
The purified natural gas is | The purified natural gas is next refrigerated and distilled to recover ethane (C<sub>2</sub>H<sub>6</sub>), propane (C<sub>3</sub>H<sub>8</sub>), butanes (C<sub>4</sub>H<sub>10</sub>) and any higher boiling hydrocarbons, collectively referred to as natural gas liquids (NGL). The natural gas is then [[condensation|condensed]] into a liquid at essentially [[atmospheric pressure]] by using further refrigeration to cool it to approximately -162 °C (260 °F). | ||
The reduction in volume makes it much more cost efficient to transport over long distances where pipelines do not exist. Where moving natural gas by pipelines is not possible or economical, it can be transported by specially designed [[Cryogenics|cryogenic]] sea vessels ([[LNG carrier]]s) or cryogenic road tankers. | The reduction in volume makes it much more cost efficient to transport over long distances where pipelines do not exist. Where moving natural gas by pipelines is not possible or economical, it can be transported by specially designed [[Cryogenics|cryogenic]] sea vessels ([[LNG carrier]]s) or cryogenic road tankers. | ||
==History== | |||
{{Image|LNG tanker Abuja.jpg|right|250px|LNG tanker with 5 spherical LNG tanks. Total length is 285 metres (311 yards).}} | |||
{{Image|LNG Terminal.jpg|right|325px|LNG storage tanks in LNG terminal at Yokohama, Japan.}} | |||
==References== | ==References== | ||
{{reflist}} | {{reflist}} | ||
Revision as of 19:44, 19 February 2011
Liquefied natural gas or LNG is natural gas (predominantly methane, CH4) that has been converted into liquid form for ease of transport and storage.
A typical raw natural gas contains only about 80% methane and a number of higher boiling hydrocarbons as well as a number of impurities. Before it is liquefied, it is typically purified so as to remove the higher-boiling hydrocarbons and the impurities. The resultant liquefied natural gas contains about 95% or more methane and it is a clear, colorless and essentially odorless liquid which is neither corrosive nor toxic.[1]
LNG occupies only a very small fraction (1/600th) of the volume of natural gas and is therefore more economical to transport across large distances. It can also be stored in large quantities that would be impractical for storage as a gas.
Process description for the production of LNG
The liquefaction process involves separating the raw natural gas from any associated water and high-boiling hydrocarbon liquids (referred to as natural gas condensate) that may be associated with the raw gas. The raw gas is then further purified in a natural gas processing plant to remove remove impurities such as the acid gases hydrogen sulfide (H2S) and carbon dioxide (CO2), any residual water liquid or vapor, mercury, nitrogen and helium which could cause difficulty downstream.
The purified natural gas is next refrigerated and distilled to recover ethane (C2H6), propane (C3H8), butanes (C4H10) and any higher boiling hydrocarbons, collectively referred to as natural gas liquids (NGL). The natural gas is then condensed into a liquid at essentially atmospheric pressure by using further refrigeration to cool it to approximately -162 °C (260 °F).
The reduction in volume makes it much more cost efficient to transport over long distances where pipelines do not exist. Where moving natural gas by pipelines is not possible or economical, it can be transported by specially designed cryogenic sea vessels (LNG carriers) or cryogenic road tankers.
History
References
- ↑ Frequently Asked Questions About LNG From the website of the California Energy Commission