Lamivudine: Difference between revisions
Jump to navigation
Jump to search

imported>David E. Volk m (→External links) |
imported>David E. Volk (chem infobox) |
||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
[[Image:Lamivudine structure.jpg| | |||
{{Chem infobox | |||
|align=right | |||
|image=[[Image:Lamivudine structure.jpg|center|thumb|200px]] | |||
|width=200px | |||
|molname=lamivudine | |||
|synonyms= 3TC, see Brand names | |||
|molformula= C<sub>8</sub>H<sub>11</sub>N<sub>3</sub>O<sub>3</sub>S | |||
|molmass= 229.2562 | |||
|uses=HIV | |||
|properties=cytosine-like RT inhibitor | |||
|hazards=see drug interactions | |||
|iupac= see chemistry section | |||
|casnumber=134678-17-4 | |||
}} | |||
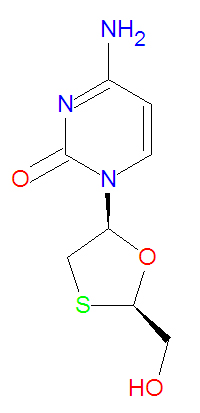
'''Lamivudine''' is a nucleotide-based [[reverse transcriptase inhibitor]] used to treat [[HIV]]/[[AIDS]] and [[hepatitis B virus]]. It is an analog of [[zalcitabine]] that competes, in the triphosphate form, for incorporation in viral DNA with [[cytosine triphosphate]]. Lacking a 3'-OH group, it terminates DNA chain elongation because the phosphodiester bond necessary for elongation cannot be made. | '''Lamivudine''' is a nucleotide-based [[reverse transcriptase inhibitor]] used to treat [[HIV]]/[[AIDS]] and [[hepatitis B virus]]. It is an analog of [[zalcitabine]] that competes, in the triphosphate form, for incorporation in viral DNA with [[cytosine triphosphate]]. Lacking a 3'-OH group, it terminates DNA chain elongation because the phosphodiester bond necessary for elongation cannot be made. | ||
Its IUPAC name is 4-amino-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2-one and it has chemical formula C<sub>8</sub>H<sub>11</sub>N<sub>3</sub>O<sub>3</sub>S. | Its IUPAC name is 4-amino-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2-one and it has chemical formula C<sub>8</sub>H<sub>11</sub>N<sub>3</sub>O<sub>3</sub>S, giving it a molecular mass of 229.2562 g/mol. | ||
Revision as of 11:41, 24 March 2008
|
| |||||||
| lamivudine | |||||||
| |||||||
| Uses: | HIV | ||||||
| Properties: | cytosine-like RT inhibitor | ||||||
| Hazards: | see drug interactions | ||||||
| |||||||
Lamivudine is a nucleotide-based reverse transcriptase inhibitor used to treat HIV/AIDS and hepatitis B virus. It is an analog of zalcitabine that competes, in the triphosphate form, for incorporation in viral DNA with cytosine triphosphate. Lacking a 3'-OH group, it terminates DNA chain elongation because the phosphodiester bond necessary for elongation cannot be made.
Its IUPAC name is 4-amino-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]pyrimidin-2-one and it has chemical formula C8H11N3O3S, giving it a molecular mass of 229.2562 g/mol.
Brand names
single drug brand names
- 3TC
- Epivir
- Epivir-HBV
- Hepitec
- Heptovir
- Zeffix
brand mixtures
- Combivir (Lamivudine + Zidovudine)
- Kivexa (Abacavir (Abacavir Sulfate) + Lamivudine)
- Trizivir (Abacavir (Abacavir Sulfate) + Lamivudine + Zidovudine)
External links
- Lamivudine - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Template:MedMaster
- Template:DrugBank