Chikungunya Virus: Difference between revisions
imported>Meg Taylor No edit summary |
Pat Palmer (talk | contribs) (fixing link to Fahrenheit (unit)) |
||
| Line 9: | Line 9: | ||
SPECIES: Chikungunya virus}} | SPECIES: Chikungunya virus}} | ||
== Description and significance == | == Description and significance == | ||
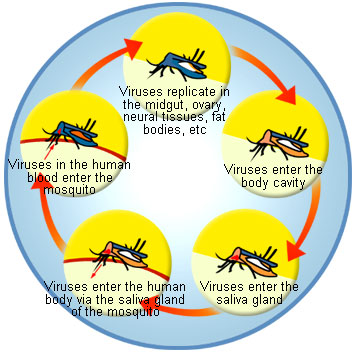
'''Chikungunya virus''' is an arthropod borne virus from the genus Alphavirus and family Togaviridae. It was first isolated in Tanzania, Africa in 1953 and has sporadically caused human epidemics in South-east Asia, southern India. Chikungunya is a zoonotic virus with a life cycle that principally involves primates and the ''Aedes'' mosquitoes. Humans that are infected may develop relatively high viremias and fever that can elevate up to 104 degrees [[Fahrenheit]]. ''Aedes aegypti'' (also called the yellow fever mosquito) is the primary transmission agent for Chikungunya. It is usually found in tropics and that is the reason it is predominantly seen in Asian countries. Chikungunya has also spread across the globe in recent years and remains a viral disease with no anti-viral medication available. The clinical picture is characterized by a sudden onset of fever, rash and severe pain in the joints. The name Chikungunya comes from the Swahili language for stooped walk, reflecting the physique of a person suffering from the disease. Chikungunya fever is also called the “bone-breaker fever”. The family Togaviridae includes viruses like O’nyong nyong and Igba Oro, which are closely related to Chikungunya and induce the same disease in humans.<ref>ProMED. 29 September 2006. V2006#437, 2006a.</ref> | '''Chikungunya virus''' is an arthropod borne virus from the genus Alphavirus and family Togaviridae. It was first isolated in Tanzania, Africa in 1953 and has sporadically caused human epidemics in South-east Asia, southern India. Chikungunya is a zoonotic virus with a life cycle that principally involves primates and the ''Aedes'' mosquitoes. Humans that are infected may develop relatively high viremias and fever that can elevate up to 104 degrees [[Fahrenheit (unit)|Fahrenheit]]. ''Aedes aegypti'' (also called the yellow fever mosquito) is the primary transmission agent for Chikungunya. It is usually found in tropics and that is the reason it is predominantly seen in Asian countries. Chikungunya has also spread across the globe in recent years and remains a viral disease with no anti-viral medication available. The clinical picture is characterized by a sudden onset of fever, rash and severe pain in the joints. The name Chikungunya comes from the Swahili language for stooped walk, reflecting the physique of a person suffering from the disease. Chikungunya fever is also called the “bone-breaker fever”. The family Togaviridae includes viruses like O’nyong nyong and Igba Oro, which are closely related to Chikungunya and induce the same disease in humans.<ref>ProMED. 29 September 2006. V2006#437, 2006a.</ref> | ||
== Natural host == | == Natural host == | ||
Revision as of 08:12, 7 December 2022
| Chikungunya Virus | ||||||
|---|---|---|---|---|---|---|
 | ||||||
| Virus classification | ||||||
|
Description and significance
Chikungunya virus is an arthropod borne virus from the genus Alphavirus and family Togaviridae. It was first isolated in Tanzania, Africa in 1953 and has sporadically caused human epidemics in South-east Asia, southern India. Chikungunya is a zoonotic virus with a life cycle that principally involves primates and the Aedes mosquitoes. Humans that are infected may develop relatively high viremias and fever that can elevate up to 104 degrees Fahrenheit. Aedes aegypti (also called the yellow fever mosquito) is the primary transmission agent for Chikungunya. It is usually found in tropics and that is the reason it is predominantly seen in Asian countries. Chikungunya has also spread across the globe in recent years and remains a viral disease with no anti-viral medication available. The clinical picture is characterized by a sudden onset of fever, rash and severe pain in the joints. The name Chikungunya comes from the Swahili language for stooped walk, reflecting the physique of a person suffering from the disease. Chikungunya fever is also called the “bone-breaker fever”. The family Togaviridae includes viruses like O’nyong nyong and Igba Oro, which are closely related to Chikungunya and induce the same disease in humans.[1]
Natural host
The virus is transmitted by infected humans to Aedes aegypti and Aedes albopictus. Virus infects during its life cycle arthropod and vertebrate hosts. Viral host belongs to the Domain Eucarya, Kingdom Animalia, Phylum Arthropoda and Chordata, Subphylum Hexapoda; Class Insecta; Subclass Pterygota; Order Diptera.
Severity and occurrence of the disease
Infection can affect the nervous system or the musculo-skeletal system. General symptoms include fever, headache, maculopapular rash, arthralgia, myalgia, photophobia, lymphadenopathy. Infection is usually acute or chronic, more than 12% of patients with Chikungunya develop chronic joint pain. Prevalence of viral infection is seasonally dependent and incidences of the virus are usually noticeable in summer and wet seasons. The incubation period lasts usually two to four days followed by a recovery in five to seven days. The virus can be detected during the first 48 hours of disease and maybe detected as late as four days in some patients. The virus is known to occur in tropical regions. Viral host lives under aerobic conditions and lives in the atmosphere where it is wet. During 2005-2006 twelve cases of Chikungunya fever were diagnosed in the United States (ProMED 2006a). In India there have been at least hundred thousand cases reported with two confirmed fatalities. Infections may occur in areas that are not considered endemic but, travel and globalization increases the possibility of epidemic outbreaks in other regions around the globe. Below is a list of some Chikungunya outbreaks:
• Tanzania in 1953 (first recoded outbreak)[2]
• Kolkata, India in 1963
• Port Klang, Malaysia in 1999
• 237 deaths and 33% of people infected in Reunion islands in 2006-2007[3]
• Italy in 2007
• Kerala, India in 2007
Genome structure
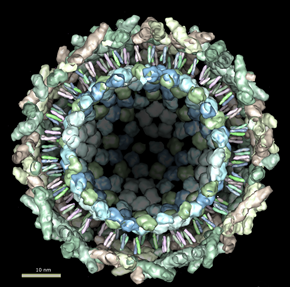
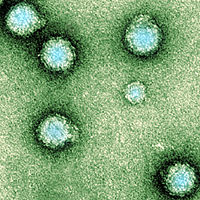
The virion consists of an envelope and a nucleocapsid. The genome consists of a single-stranded, positive-sense RNA molecule of approximately 12000 nucleotides long. The 5’ end is capped with a 7-methylguanosine while the 3’ end is polyadenylated. A subgenomic positive-strand RNA referred to as 26SRNA is transcribed from a negative-stranded RNA intermediate. This RNA serves as the mRNA for the synthesis of the viral structural proteins. Alphaviruses have conserved domains that play an important role in the regulation of viral RNA synthesis.[4] These domains are found at the 5’ and 3’ ends as well as at the intergenic region. Virions are spherical and measure about 70nm in diameter. Surface projections are glycoprotein spikes covering the surface evenly.
Genome organization and replication
Virions located on the surface of the cell membrane enter the host cells by fusion and endocytosis of the viral envelope. The uncoating of the virions occurs in the cytoplasm. The site of mRNA transcription is in the cell cytoplasm. Replication is not restricted to a particular tissue or organ of the host so the virus replication occurs in various organs. The insect host initiates the virus replication. The genome replication is done in the cytoplasm.[5]
Diagnosis
The best way to diagnose the disease is by distinguishing the Chikungunya strain by kinetic hemaglutination-inhibition tests. Even monoclonal antibodies can be used in test such as the ELISA. The ELISA, Enzyme-linked immunosorbent assay, is a technique used in immunology to detect presence of an antibody or an antigen in a blood sample. Recently, a reverse transcriptase PCR technique has also been used for the diagnosis of Chikungunya virus. PCR results can be available within one to two days.
Prevention
Currently there is no prevention for this virus causing disease. There are no anti-viral medications available although ones own immune system is the best fighter for this virus. Mosquito repellents can be used to avoid from getting bitten and wearing clothing that is covering your extremities is a big help. Eliminating mosquito-breeding sites is another key prevention measure. The mosquito bites in the day time and reduces its humming of the wings while it is approaching the target and it will bite from beneath the arms.[6]
Phylogenetic relationships
There are very few reports on the molecular relationship between Chikungunya and other members of the Alphavirus genus. It is very closely related to the O’nyong nyong virus. Chikungunya and O’nyong nyong virus have 85% similarities in their genome.
Current research
First article: How Chikungunya has spread to new vectors and new locations?
Researchers from the university of Texas Medical Branch have discovered how a key protein switch allows Chikungunya virus to spread to new vectors. Since Chikungunya virus cases have increased globally in the recent years this study also focused on another types of vector that carries this virus. It was spotted in the Indian Ocean islands where Aedes aegypti the primary carrier is not found. In this area another vector named Aedes albopictus, a relative of the Asian tiger mosquito was found carrying the virus. In earlier studies done from the epidemic on islands of the Indian Ocean the Chikungunya strain had enveloped protein gene, E1-A226V. The researchers cloned the virus and infected the aedes aegypti and the aedes albopictus with two genetically engineered clone of the virus, one had mutation and the other did not. The transmission of the mutant virus was much greater than the other virus in aedes albopictus. This proved that E1-A226V is directly responsible for Chikungunya adaptation to the aedes albopictus. The Asian tiger mosquito is spreading in Europe and the United States and interestingly since there is a global warming increment there could be an increase in new geographic locations for the Chikungunya virus to infect.[7]
Second article: Biomarkers detected for Chikungunya virus
This study was performed at Singapore’s Tan Tock Seng Hospital. In this study researchers investigated many biological factors such as cytokines and chemokines that were produced in the human blood. Cytokines are proteins, peptides or glycoprotein's that are signaling molecules that like hormones and neurotransmitters are used in cellular communication. Chemokines on the other hand are small cytokines with low molecular weight that are also released by many cells. There are three specific biological factors that were distinguished in patients with the severe form of the disease, interleukin-1 beta, interleukin-6 and RANTES that is a protein. RANTES is an acronym for Regulated on Activation, Normal T Expressed and Secreted. The study was conducted on blood samples obtained from ten patients who developed the disease during the Singapore’s Chikungunya virus outbreak in January 2008. Lisa Ng, Ph.D., principal investigator of the Chikungunya research team at SIgN and co-author of the PLoS ONE article, said, "This first comprehensive report, which examines the cellular signals produced as part of the human immune response to Chikungunya virus infection, enables us to understand the changes in molecular signals in the body when infection sets in. These biomarkers can potentially lead to the development of therapeutics to reduce the severity of the disease and halt its progression." Severe form of Chikungunya was indicated decrease in the levels RANTES but an increased in the levels of interleukin-1 beta, interleukin-6.[8] This study proves that cytokines could be used as biomarkers in predicting the severity of the disease since they provide immunological information to understand the effect of Chikungunya in the human host.[9]
Third article: Connective tissue metabolism in Chikungunya patients
This study was done on 75 Chikungunya virus infected patients. Since it causes severe arthritis in human hosts by a large area of necrosis and collagenosis or fibrosis. In this research the connective tissue was the main focus since the virus damages the cartilage and increases the levels of proline, mucopolysaccharide, and hydroxyproline measurements of the infected hosts. These tests are done through urine analyses to check if there is a presence of mucoplysaccharides in the urine. The study had results with moderate to severe cases. Since the connective tissue metabolism was greatly increased due to the infection, there was proline, hydroxyproline and mucopolysaccharides present in the urine. This indicated that the patients that were infected by the virus had damage done to their cartilage and connective tissue which had particles excreted through their urine.[10]
Application to biotechnology
The use of Chikungunya Virus in biotechnology is currently unavailable.
References
- ↑ ProMED. 29 September 2006. V2006#437, 2006a.
- ↑ 'Chikungunya', Disease Surveillance and Epidemiology
- ↑ 'Chikungunya in La Réunion Island (France)', Global Alert and Response (GAR)
- ↑ Strauss, J. H. & Strauss, E. G. (1988). Evolution of RNA viruses. Annual Review of Microbiology 42, 657-683.[Medline]
- ↑ 'Chikungunya virus', ICTVdB, 00.073.0.01.007
- ↑ 'Prevention of Chikungunya Virus Infection', Chikungunia
- ↑ 'How Chikungunya Virus Has Spread To New Vectors And Locations', Science Daily, 10 December 2007
- ↑ 'RANTES', MedicineNet
- ↑ 'Biomarkers detected for Chikungunya fever', Phys.org, 4 March 2009
- ↑ 'Connective tissue metabolism in chikungunya patients', Virol J. 2008; 5: 31. Published online 2008 February 27. doi: 10.1186/1743-422X-5-31