Bacteriophage/Citable Version: Difference between revisions
John Leach (talk | contribs) m (Text replacement - "[[Ganges" to "[[River Ganges") |
Pat Palmer (talk | contribs) m (Text replacement - "River Ganges" to "Ganges River") |
||
| Line 8: | Line 8: | ||
==History== | ==History== | ||
In 1896, [[M. E. Hankin]] reported that ''something'' in the waters of the [[River | In 1896, [[M. E. Hankin]] reported that ''something'' in the waters of the [[Ganges River]] and [[Jumna]] rivers in India had marked [[antibacterial]] action against the bacteria responsible for the disease, [[cholera]]. Testing these waters showed that this inhibition of the bacterial growth remained even if the water was passed through a very fine porcelain filter.<ref>Adhya S, Merril C (2006) The road to phage therapy. Nature 443: 754-755 doi:10.1038/443754a</ref> That meant that whatever was responsible for killing off the ''Vibrio cholera'' cultures was smaller than any known bacterium, none of which could pass through the tiny pores present in that dense ceramic filter. | ||
In 1915, [[Frederick Twort]], a British [[physician]] and [[microbiology|bacteriologist]], described bacteriophages (without calling them that) discovered in an unusual "glassy transformation" of certain bacterial colonies he had grown in his laboratory. That laboratory, and his ability to do independent research in it, was a perk at his post of Superintendent at Brown Institution, London University (a veterinary hospital). Some sort of material produced by these bacterial colonies killed them off, and it was Twort who showed that these agents were particles so small that they could pass through a porcelain filter. Further, they multiplied while prompting the bacterial cultures to die. Realizing that this agent must be smaller than any known bacterium, he suspected that it might be either 1) a stage in the life cycle of some bacteria, 2) a chemical [[enzyme]] produced by the bacteria, or 3) a virus that grows on and destroys the bacteria.<ref>Twort F.W (1915) An investigation on the nature of ultra-microscopic viruses. Lancet 2:1241-3</ref> Twort's work was interrupted by the onset of [[World War I]], but when he returned to the [[Brown Institution]], he spent the rest of his career trying to grow this agent on artificial media of the kind that support bacterial growth. Of course, bacteriophages are viruses, and, like all viruses, unbeknownst to him, can only "live" in cells rather than on artificial culture media, and so his research could never succeed. However, he did conclude that filterable particles killed bacteria — and increased their own numbers in the process of doing so: the essential actions of the bacteriophage. | In 1915, [[Frederick Twort]], a British [[physician]] and [[microbiology|bacteriologist]], described bacteriophages (without calling them that) discovered in an unusual "glassy transformation" of certain bacterial colonies he had grown in his laboratory. That laboratory, and his ability to do independent research in it, was a perk at his post of Superintendent at Brown Institution, London University (a veterinary hospital). Some sort of material produced by these bacterial colonies killed them off, and it was Twort who showed that these agents were particles so small that they could pass through a porcelain filter. Further, they multiplied while prompting the bacterial cultures to die. Realizing that this agent must be smaller than any known bacterium, he suspected that it might be either 1) a stage in the life cycle of some bacteria, 2) a chemical [[enzyme]] produced by the bacteria, or 3) a virus that grows on and destroys the bacteria.<ref>Twort F.W (1915) An investigation on the nature of ultra-microscopic viruses. Lancet 2:1241-3</ref> Twort's work was interrupted by the onset of [[World War I]], but when he returned to the [[Brown Institution]], he spent the rest of his career trying to grow this agent on artificial media of the kind that support bacterial growth. Of course, bacteriophages are viruses, and, like all viruses, unbeknownst to him, can only "live" in cells rather than on artificial culture media, and so his research could never succeed. However, he did conclude that filterable particles killed bacteria — and increased their own numbers in the process of doing so: the essential actions of the bacteriophage. | ||
Latest revision as of 12:50, 8 March 2024
Version 1, 22 August 2013, updated links 3 August 2018
A bacteriophage ("bacteria eater", from 'bacteria' and Greek φαγειν, "to eat") is a virus that infects bacteria. The term is most commonly used in its shortened form: phage. The bacteriophage was first shown to be a part of the biological world in modern times, less than a hundred years ago. The germ theory of disease, itself, has been a concept only since the 19th century, and brought such a novel understanding of how infectious diseases are spread that Medicine experienced revolutionary advances with its acceptance. The surprising conclusion that germs themselves can also have germs only came after techniques used in the discovery of viruses in the early 20th century were also used to study some puzzling observations concerning the ailments of locusts, the culture of bacteria, and the sacred waters of the river Ganges.
As it turns out, bacteria are often infected with viruses called bacteriophages, and play "host" to them just as the bodies of human beings, plants, and other animals host infections of both bacteria and viruses. Phages are ubiquitous and can be found in all habitats populated by the bacterial cells that these viruses need to enter in order to be able to reproduce. Those habitats are incredibly diverse, and include the soil, the intestines of animals, and the sea. Scientists look to these habitats to find phages for study.
One of the richest natural sources is sea water, where up to 107 phages per ml (or, at least, virus-like particles)[1] can be found. Whitman et al. argue that there are between 1030 and 1031 prokaryotic cells on our planet.[2] If we assume that there is one virus for every prokaryote cell (and bear in mind most bacterial cells tested are found to harbor latent phage (prophage)), then we conservatively reach a total population of 1030 to 1032 of these virus-like particles in the world.[3]
History
In 1896, M. E. Hankin reported that something in the waters of the Ganges River and Jumna rivers in India had marked antibacterial action against the bacteria responsible for the disease, cholera. Testing these waters showed that this inhibition of the bacterial growth remained even if the water was passed through a very fine porcelain filter.[4] That meant that whatever was responsible for killing off the Vibrio cholera cultures was smaller than any known bacterium, none of which could pass through the tiny pores present in that dense ceramic filter.
In 1915, Frederick Twort, a British physician and bacteriologist, described bacteriophages (without calling them that) discovered in an unusual "glassy transformation" of certain bacterial colonies he had grown in his laboratory. That laboratory, and his ability to do independent research in it, was a perk at his post of Superintendent at Brown Institution, London University (a veterinary hospital). Some sort of material produced by these bacterial colonies killed them off, and it was Twort who showed that these agents were particles so small that they could pass through a porcelain filter. Further, they multiplied while prompting the bacterial cultures to die. Realizing that this agent must be smaller than any known bacterium, he suspected that it might be either 1) a stage in the life cycle of some bacteria, 2) a chemical enzyme produced by the bacteria, or 3) a virus that grows on and destroys the bacteria.[5] Twort's work was interrupted by the onset of World War I, but when he returned to the Brown Institution, he spent the rest of his career trying to grow this agent on artificial media of the kind that support bacterial growth. Of course, bacteriophages are viruses, and, like all viruses, unbeknownst to him, can only "live" in cells rather than on artificial culture media, and so his research could never succeed. However, he did conclude that filterable particles killed bacteria — and increased their own numbers in the process of doing so: the essential actions of the bacteriophage.
Independently, the French-Canadian microbiologist Félix d'Hérelle, working at the Pasteur Institute in Paris, announced on September 3, 1917 that he had discovered "an invisible, antagonistic microbe of the dysentery bacillus"[6] For d’Herelle, there was no question as to the nature of his discovery: "In a flash I had understood: what caused my clear spots was in fact an invisible microbe... a virus, parasitic on bacteria." D'Herelle called the virus bacteriophage. His discovery came about from culturing a dysentery bacillus from a patient at the hospital whose particular strain of pathogenic bacteria was infected with a phage. D'Herelle noted that filtering the cultures of the bacteria that showed clearing, and adding this filtrate to vibrantly growing cultures of the same species of bacteria, completely killed off the bacterial population. He correlated this finding with the patient's own recovery, which he attributed to the antibacterial action of the phage, and to his past work with locusts. That work had first shown him the phenomenon of a transferable agent that was able to clear away areas of growth in bacterial cultures. Years previously, he had studied a bacterial diarrhea of locusts that was so severe that spreading this disease was useful in the agricultural control of the insects. At that time, when he had cultured the bacteria from the insects' abnormal waste products, he found that some of the cultures had odd clear spots. Although he had also discovered, at that time, that the agent responsible for clearing the bacteria could be transferred from one culture to another, he was not sure of the significance of these findings. Perhaps it was this agent that was itself responsible for the disease in locusts, and the bacteria from their guts might only be a coincidental isolate. Later, with his study of bacteria from human dysentery, as just noted, he realized that instead the agents responsible for clearing actually were antagonistic to the cause of the diarrhea, the cause in both the human and insect cases being the dysentery bacillus.[7] Duckworth (1976) provides a detailed historical account of the discovery of bacteriophages.[8]
Major discoveries with phages
The early history of bacteriophages might suggest that their study would immediately be applied to the treatment of diseases caused by bacteria. This was not the case; instead antibiotic therapy became the mainstay of treatment for bacterial diseases. However, the importance of the bacteriophage in the advancement of biological science cannot be overstated. Bacteriophages were intensively studied in the decades after their discovery, and came to play a leading role in the advancement of the basic science of microbiology and the new biology of molecular genetics. In the 1940's, and onward, it was the laboratory study of phage biology that directly yielded major insights into bacterial genetics, molecular biology, and the exact manner in which viruses reproduce and spread. These discoveries include:
- Mutations arise in the absence of selection (Luria and Delbruck 1943)[9]
- genetic transduction (Zinder and Lederberg 1952)[10]
- DNA is genetic material ((Hershey and Chase 1952)[11]
- restriction and modification ((Luria and Human 1952; Dussoix and Arber 1962)
- genetic fine structure ((Benzer 1955)[12]
- messenger RNA ((Volkin and Astrachan 1956)[13]
- acquisition and loss of genes from genomes ((Campbell 1962)[14]
- molecular basis of DNA recombination ((Meselson and Weigle 1961)[15]
- gene regulation ((Jacob and Monod 1961)[16]
- triplet nature of DNA code (Crick, Barnett, Brenner and Watts-Tobin 1961)[17]
- DNA and protein are colinear (Sarabhai, Stretton, Brenner and Bolle 1964)[18]
- DNA ligase (Gellert 1967)[19]
- DNA recognition and cooperative binding (Ptashne 1967)[20]
- chaperones and protein folding (Georgeopoulos, Hendrix, Casjens and Kaiser 1973)
- first DNA genome sequenced, phiX174 (Sanger et al. 1977)
- epigenetic gene regulation (Ptashne 2004)
- repression and activation (i.e. turning genes on and off)[21]
Structure
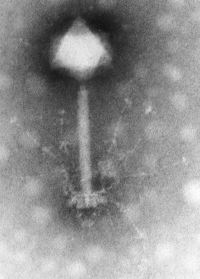
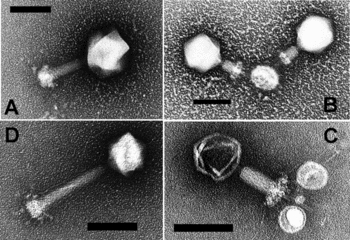
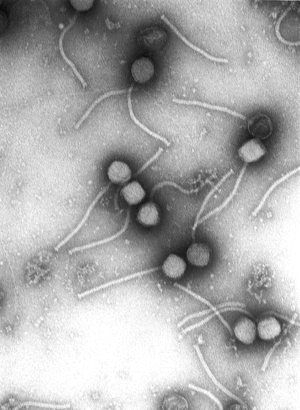
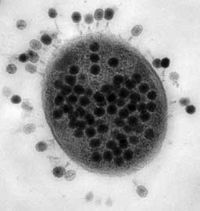
1. Size - Most phages are 24–200 nm in length.(A typical bacterium cell may be about 1000 nm long, which in turn is 1/1000th of a millimeter = 1 micron in length.)
2. Head or Capsid - Every phage has a head structure, which can vary in size and shape. Some are icosahedral (20 sides), others are filamentous. The head (or capsid) is composed of many copies of one or more type of protein, and it contains the phage's genetic material (i.e. nucleic acid). The genetic material can be ssRNA, dsRNA, ssDNA, or dsDNA between 5 and 500 kilo base pairs (kbp) long in either a circular or linear arrangement.
3. Tail - Many, but not all, phages have tails attached to the phage head. The tail is a hollow tube through which the nucleic acid passes during infection, and its size can vary considerably. In the more complex phages, like T4, the tail is surrounded by a contractile sheath which contracts during infection of the bacterium. At the end of the tail, some phages have a base plate and one or more tail fibers attached to it; these structures are involved in the attachment of the phage to the bacterial cell.
Classification
An alphabetical list of the bacteriophage families:
Corticoviridae icosahedral capsid with lipid layer, circular supercoiled dsDNA
Cystoviridae enveloped, icosahedral capsid, lipids, three molecules of linear dsRNA
Fuselloviridae pleomorphic, envelope, lipids, no capsid, circular supercoiled dsDNA
Inoviridae genus Inovirus long filaments with helical symmetry, circular ssDNA
Inoviridae genus Plectrovirus short rods with helical symmetry, circular ssDNA
Leviviridae quasi-icosahedral capsid, one molecule of linear ssRNA
Lipothrixviridae enveloped filaments, lipids, linear dsDNA
Microviridae icosahedral capsid, circular ssDNA
Myoviridae, A1 tail contractile, head isometric
Myoviridae, A2 tail contractile, head elongated (length/width ratio = 1.3-1.8)
Myoviridae, A3 tail contractile, head elongated (length/width ratio = 2 or more)
Plasmaviridae pleomorphic, envelope, lipids, no capsid, circular supercoiled dsDNA
Podoviridae, C1 tail short and noncontractile, head isometric
Podoviridae, C2 tail short and noncontractile, head elongated (length/width ratio = 1.4)
Podoviridae, C3 tail short and noncontractile, head elongated (length/width ratio = 2.5 or more)
Rudiviridae helical rods, linear dsDNA
Siphoviridae, B1 tail long and noncontractile, head isometric
Siphoviridae, B2 tail long and noncontractile, head elongated (length/width ratio = 1.2-2)
Siphoviridae, B3 tail long and noncontractile, head elongated (length/width ratio = 2.5 or more)
Replication
Bacteriophages are obligate parasites, and most undergo a lytic lifecycle. To propagate, they must enter bacterial host cells. Once inside a host cell, phages hijack the host cell's DNA replication machinery and induce the host to manufacture more phages. When enough phage offspring are produced (usually ~100), host cells will be broken open (lysed) and die. After lysis, the phage offspring spill out of the cell and diffuse toward new hosts.
Attachment
To enter a host cell, bacteriophages attach to specific receptors on the surface of bacteria, including lipopolysaccharides, teichoic acids, proteins or even flagella. In other words, a phage can only enter a cell through a particular feature that it "recognizes", and is not able to infect a bacterium that lacks the inviting feature. Each type of phage has only a certain set of features that it recognizes, and so not all phages are able to infect all bacteria. In this way phages are said to have "specificity" for the bacteria that they do infect. This specificity means that a bacteriophage can only enter bacteria that bear the certain types of receptors that they can bind to, and it is these portals of entry that determine the phage's "host range". As phage virions do not move, they rely on random encounters with the right receptors when in solution (e.g., water, blood or lymphatic circulation). Complex bacteriophages, such as the T-even phages, are thought to use a syringe-like motion to inject their genetic material into the cell. After making contact with the appropriate receptor, the tail fibers bring the base plate closer to the surface of the cell. Once it is attached completely, conformational changes in the proteins cause the tail to contract, possibly with the help of ATP present in the tail.[22]
Synthesis of proteins and nucleic acid
Within a short time, sometimes just minutes, bacterial ribosomes start translating viral mRNA into protein. For RNA-based phages, RNA replicase, the enzyme catalyst involved in genetic replication, is synthesised early in the process. Early proteins and a few proteins that were present in the virion may modify the bacterial RNA polymerase so that it preferentially transcribes viral mRNA. The host’s normal synthesis of proteins and nucleic acids is disrupted, and it is forced to manufacture viral products. These products go on to become part of new virions within the cell, helper proteins which help assemble the new virions, or proteins involved in cell lysis.
Virion assembly
In the case of the T4 phage, the construction of new virus particles is a complex process which requires the assistance of special 'helper' molecules. The base plate is assembled first, with the tail being built upon it afterwards. The head capsid, constructed separately, will spontaneously assemble with the tail. The DNA is packed efficiently within the head in a manner which is not yet known. The whole process takes about 15 minutes.
Release of virions
Phages may be released via cell lysis or by host cell secretion. In T4 phage, upwards of three hundred phages will be released via lysis in approximately twenty minutes after injection. Host lysis is usually achieved through an enzyme called endolysin which attacks and breaks down the cell wall tructure surrounding the bacterial cell which is composed of a sugar-amino acid co-polymer called peptidoglycan. Some phages, such as Lambda phage of Escherichia coli, also use an additional protein, called holin, to make lesions in the host's cytoplasmic membrane.
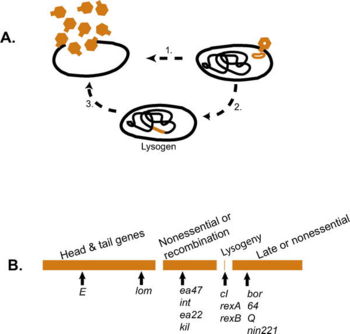
Lysogenic life cycle
Some phages, such as the phage Lambda, undergo a second type of life cycle, called the lysogenic cycle, in addition to the lytic cycle. By contrast to the lytic cycle, the lysogenic cycle does not result in host cell lysis. Phages able to undergo lysogeny are known as temperate phages. Their viral genome will integrate with host DNA and replicate along with it fairly harmlessly, or may even become established as a plasmid. Thus, the host cell continues to survive and reproduce, and the hitchhiking phage is reproduced in all of the cell’s offspring. Sometimes prophages may provide benefits to the host bacterium while they are dormant by adding new functions to the bacterial genome in a phenomenon called lysogenic conversion. A famous example is the conversion of a harmless strain of Vibrio cholerae by a phage into a highly virulent one, which causes cholera.
In general, when host cells are actively growing and the environmental conditions are favorable, temperate phages will use the lytic pathway. When environmental conditions worsen, temperate phages tend to enter the lysogenic pathway (Ptashne 2004). This is plausible because starved host cells may not have the components required for phage reproduction; moreover, salient environmental conditions may not be auspicious for phage survival outside the cell. It may be more advantageous to remain in the host than to embark out into a hostile environment.
Phage ecology
Phage ecology is the study of the interaction of bacteriophages with their environments. Traditional phage biology focused mainly on the characterization of the bacteriophage's biochemistry and molecular biology (see Stent 1963). Recent work has focused more on the bacteriophage's role in the environment, particularly with respect to phage organismal, population, community and ecosystem ecology.[23] An active phage ecology clearinghouse is available at www.phage.org.
Phage therapy
Frederick Twort promoted the use of phages as anti-bacterial agents soon after their discovery, but antibiotics, upon their discovery, proved more practical. Research on phage therapy was largely discontinued in the West, but phage therapy has been used since the 1940s in the former Soviet Union as an alternative to antibiotics for treating bacterial infections.
The evolution of bacterial strains through natural selection that are resistant to multiple drugs has led some medical researchers to re-evaluate phages as alternatives to antibiotics. Unlike antibiotics, phages can co-evolve with the bacteria, as they have done for millions of years, so a sustained resistance is unlikely.
As most phage strains can infect a limited range of bacterial hosts (ranging from several species to only certain subtypes within a species), phage therapy must be carefully tailored to host presence. This can be an advantage because no other bacteria are attacked, making the therapy work similarly to a narrow-spectrum antibiotic. It can also be a disadvantage in infections with several different types of bacteria, which is often the case. Sometimes mixes of several strains of phage are used to create a broader spectrum cure. Another problem with bacteriophages is that they are attacked by the body's immune system.
Phages work best when in direct contact with the infection, so they are often applied directly to an open wound. This is rarely applicable in the current clinical setting where infections occur systemically. Despite individual success in the former Soviet Union, many researchers studying infectious diseases question whether phage therapy will achieve any medical relevance. There have been no large clinical trials to test the efficacy of phage therapy yet, but research continues because of the rise of multiple antibiotic resistance.
The George Eliava Institute of Bacteriophage, Microbiology and Virology in Tblisi, Georgia (country) is a leading laboratory conducting research on phage therapy.
Other uses for bacteriophages
Food safety
In August, 2006 the United States Food and Drug Administration (FDA) approved using bacteriophages on certain ready-to-eat meats to kill the potentially lethal Listeria monocytogenes bacteria. The additive, known as LMP-102™, is a proprietary cocktail of six bacteriophages specific for Listeria, from Intralytix, Inc. Intralytix is also seeking FDA approval for Escherichia coli O157:H7 and Salmonella treatments.
Nanotechnology
Another large use of bacteriophages is by the company Cambrios Technologies. Its founder, Dr Angela Belcher, pioneered the use of the M13 bacteriophage to create nanowires and electrodes. She started her research by studying how abalone snails create their shells from things that naturally occur in their environment. Specifically, she discovered the snails take abalone and make them transform into two distinct crystalline structures. One of the structures was hard, the other was fast-growing. She took this concept and applied it to bacteriophages. One of her ventures consisted of implanting gold and cobalt oxide in a bacteriophage to create a paper-thin electrode: the gold was for conductivity, and the cobalt oxide was for the actual use of the battery.
Bacteriophage experimental evolution
Experimental evolution is the use of model organisms (e.g. Escherichia coli, yeast, lambda phage) to study the process of evolution in controlled experiments. Because of their rapid generation times, small sizes, ease of manipulation, small genomes and availability of molecular genetic and biochemical details, bacteriophages are ideal organisms for experimental studies of evolution. Phage experimental evolution is part of the broader field of viral evolution.
See here for an annotated bibliography of modern phage experimental evolution studies.
References
- ↑ Wommack KE, Colwell RR (2000). Virioplankton: viruses in aquatic ecosystems. Microbiol Mol Biol Rev 64:69-114
- ↑ Whitman WB et al. (1998) Prokaryotes: The unseen majority. Proc Natl Acad Sci USA 95:6578-83
- ↑ e.g. Bergh O et al. (1989) High abundance of viruses found in aquatic environments. Nature 340:467-8
- ↑ Adhya S, Merril C (2006) The road to phage therapy. Nature 443: 754-755 doi:10.1038/443754a
- ↑ Twort F.W (1915) An investigation on the nature of ultra-microscopic viruses. Lancet 2:1241-3
- ↑ d'Herelle F (1917) An invisible antagonist microbe of dysentery bacillus. Comptes Rendus Hebdomadaires des Seances de L’academie des Sciences 165:373-5
- ↑ Stent GS (1963) The Molecular Biology of Bacterial Viruses. WH Freeman & Co.
- ↑ Duckworth DH (1976) Who discovered bacteriophage? Bacteriol Rev 40:793-802
- ↑ Luria SE, Delbruck M (1943) Mutations of bacteria from virus sensitivity to virus resistance Genetics 28:491-511
- ↑ Zinder ND, Lederberg J (1952) Genetic exchange in salmonella. J Bacteriol 64: 679-99
- ↑ Hershey AD, Chase M (1952) Independent functions of viral protein and nucleic acid in growth of bacteriophage. J Gen Physiol 36:39-56
- ↑ Benzer S (1955) Fine structure of a genetic region in bacteriophage. Proc Natl Acad Sci USA 41:344–54
- ↑ Volkin E, Astrachan L (1956) Intracellular distribution of labeled ribonucleic acid after phage infection of Escherichia coli. Virology 2: 433-7
- ↑ Campbell A (1962) Episomes. Advances in Genetics 11:101-145
- ↑ Meselson M, Weigle JJ (1961) Chromosome breakage accompanying genetic recombination in bacteriophage. Proc Natl Acad Sci USA 47:857-68
- ↑ Jacob F, Monod J (1961) Genetic regulatory mechanisms in synthesis of proteins. J Mol Biol 3:318-356.
- ↑ Crick FH et al. (1961) General nature of the genetic code for proteins. Nature 192:1227-32
- ↑ Sarabhai AS et al. (1964) Co-linearity of the gene with the polypeptide chain. Nature 201:13-
- ↑ Gellert M (1967) Formation of covalent circles of lambda DNA by Escherichia coli extracts. Proc Natl Acad Sci USA 57:148-55
- ↑ Ptashne M (1967) Isolation of lambda phage repressor. Proc Natl Acad Sci USA 57:306–13
- ↑ Ptashne M (2004) A Genetic Switch: Phage Lambda Revisited. 3rd Ed. Cold Spring Harbor Laboratory Press. ISBN: 0879697172
- ↑ Prescott L (1993) Microbiology. WC Brown Publishers, ISBN 0-697-01372-3
- ↑ Goyal SM et al. (1987) Phage Ecology. John Wiley and Sons, New York. ISBN 0471824194
- Abedon ST (2006) Phage ecology. Pp. 37-46 in R. Calendar and S.T. Abedon, eds. The Bacteriophages, 2nd Edn, Oxford University Press. ISBN 0195148509
- Brussow H, Kutter E (2005) Phage ecology. Pp. 129-163 in E. Kutter and A. Sulakvelidze, eds. Bacteriophages: Biology and Applications. CRC Press. ISBN: 0849313368