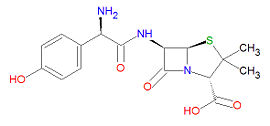
Amoxicillin
|
| |||||||
| amoxicillin | |||||||
| |||||||
| Uses: | antibiotic drug | ||||||
| Properties: | beta-lactam | ||||||
| Hazards: | see drug interactions | ||||||
| |||||||
Amoxicillin (AMX), also spelled amoxycillin, and also called p-hydroxyampicillin, is an antibiotic drug that is very similar to ampicillin but which has enhanced stability towards gastric juices compared to other -lactam antibiotics. It is effective against a wide range of Gram-positive bacteria but limited Gram-negative bacteria. It is used for bacterial strains that do not produce -lactamase, an enzyme which would degrade the drug. The incidence of -lactamase-resistence in bacteria is increasing, leading to the increased use of clavulanic acid, a -lactamase inhibitor, with amoxicillin and other -lactam drugs. It is used to treat infections in the ears, nose and throat, the genitourinary tract, the skin and the respiratory tract. It is effective against E. coli, S. pneumoniae, Staphylococcus spp., H. influenzae, P. mirabilis, E. faecalis, N. gonorrheae and some strains of Streptococcus spp..
Chemical properties
Its IUPAC chemical name is (2S,5R,6R)-6-[[(2R)-2-amino-2-(4-hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid and its chemical formula is C16H19N3O5S, giving it a molecular mass of 365.4042 g/mol. It is a beta-lactam drug and is hence susceptible to degradation by -lactamase enzymes.
Mechanism of action
Amoxicillin binds to the penicillin-binding protein 1A (PBP-1A) located inside the bacterial cell well. It inhibits the last stage of bacterial cell wall synthesis by acylating the penicillin-sensitive transpeptidase C-terminal domain thereby stopping the cross-linking of peptidoglycan strands. Autolytic enzymes in the bacteria then lyse the cells.
Drug interactions
Amoxicillin may be an agonist of demeclycycline, doxycycline, methacycline, minocycline, oxytetracycline, Rolitetracycline and tetracycline. It may decrease the effects of some contraceptives, including ethinyl estradiol and mestranol, and increases the effects of methotrexate.
External links
- Amoxicillin - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Template:MedMaster
- Template:DrugBank