Vibrio cholerae
 | ||||||||||||||
| Scientific classification | ||||||||||||||
| ||||||||||||||
| Binomial name | ||||||||||||||
| Vibrio cholerae |
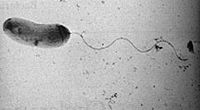
Vibrio cholerae is a Gram-negative, curved rod-shaped bacterium that has a single polar flagellum. It is an aerobic organism and thrives best in alkaline media, The organism causes a major epidemic gastrointestinal disease, cholera.
Application to Biotechnology
Vaccines for cholera are currently being investigated for further development. There are currently three licensed oral cholera vaccines avaible. They are the WC/rBS vaccine, Variant WC/rBS vaccine, and the CVD 103-HgR vaccine.
The WC/rBS vaccine contains killed whole-cell Vibrio cholerae strain O1 and purified recombinant B subunit of the cholera toxin. This vaccine was administered in Bangladesh, Peru, and Sweden where trials show that there is a 80 to 95% protection rate against cholera for six months among all age groups after administering two doses of the vaccine. However, it was found that in Bangladesh, the protection rate decreased after six months in young children but it still had a substantial protection rate of 60% in adults and older children after two years. The Variant WC/Rbs vaccine, which contains no recombinant B subunit, was produced in Vietnam where it was also administered to all age groups. Two doses were given one week apart. In 1992 to 1993, trials performed in Vietnam revealed a protection rate of 66% at eight months for all various age groups. This vaccine is licensed and available in Vietnam exclusively. The third oral vaccine for cholera is the CVD 103-HgR vaccine which contains an attenuated live strain of Vibrio cholerae strain O1 which had been genetically altered. Clinical trials were performed in the United States where there was a efficacy protection rate of approximately 95%. However in Indonesia, there was a poor level of protection because the population was exposed to cholera for an extended period of timeafter immunization had occurred.[2]
Current Research
Multivalent drug design and inhibition of cholera toxin by specific and transient protein-ligand interactions.Liu J, Begley D, Mitchell DD, Verlinde CL, Varani G, Fan E.Department of Chemistry, University of Washington, Seattle, WA 98195, USA. 2008 May;71(5):408-19. Epub 2008 Mar 27.
This research is studying how to inhibit the cholera toxin B subunit through precise and transient protein-ligand communication by creating a multivalent drug designed for this purpose. [3]
Is HIV infection associated with an increased risk for cholera? Findings from a case-control study in Mozambique. Von Seidlein L, Wang XY, Macuamule A, Mondlane C, Puri M, Hendriksen I, Deen JL, Chaignat CL, Clemens JD, Ansaruzzaman M, Barreto A, Songane FF, Lucas M. International Vaccine Institute, Seoul, Korea. Trop Med Int Health. 2008 Mar 6
In this study, researchers are focusing on the possible association between cholera and HIV infection. This study was performed in Sub-Saharan Africa where both cholera and HIV are highly abundant diseases.[4]
Does an L-glutamine-containing, glucose-free, oral rehydration solution reduce stool output and time to rehydrate in children with acute diarrhoea? A double-blind randomized clinical trial. Gutiérrez C, Villa S, Mota FR, Calva JJ. J Health Popul Nutr. 2007 Sep;25(3):278-84.
This study observed the effects of different oral rehydration solutions administered for the treatment of cholera. Instead of rehydration with glucose, it is replaced by L-glutamine (L-glutamine ORS). Researchers then compared the amount of time it took for the reduction of diarrhea and rate of rehydration between the two versions, the standard Oral Rehydration Solution ORS and L-glutamine ORS.[5]
References
- ↑ Vibrio cholerae: Genomics and Molecular Biology. Caister Academic Press Edited by Edited by: Shah M. Faruque and G. Balakrish Nair International Centre for Diarrhoeal Disease Research, Dhaka-1212, Bangladesh and National Institute of Cholera and Enteric Diseases, Beliaghata, Kolkata - 700 010, India. Published July 2008.
- ↑ http://www.who.int/topics/cholera/vaccines/current/en/index.html
- ↑ http://www.ncbi.nlm.nih.gov/pubmed/18373548?ordinalpos=5&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
- ↑ http://www.ncbi.nlm.nih.gov/pubmed/18331384?ordinalpos=11&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
- ↑ http://www.ncbi.nlm.nih.gov/pubmed/18330060?ordinalpos=12&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
http://www.genomenewsnetwork.org/articles/08_00/cholera_genome.shtml [1]
http://www.ph.ucla.edu/EPI/snow/firstdiscoveredcholera.html [5]
http://www.who.int/topics/cholera/en/ [6]
http://www.cdc.gov/nczved/dfbmd/disease_listing/cholera_gi.html [8]
http://www.who.int/topics/cholera/vaccines/current/en/index.html [9]
Liu J, Begley D, Mitchell DD, Verlinde CL, Varani G, Fan E.Multivalent drug design and inhibition of cholera toxin by specific and transient protein-ligand interactions.Department of Chemistry, University of Washington, Seattle, WA 98195, USA. 2008 May;71(5):408-19. Epub 2008 Mar 27. [10]
Von Seidlein L, Wang XY, Macuamule A, Mondlane C, Puri M, Hendriksen I, Deen JL, Chaignat CL, Clemens JD, Ansaruzzaman M, Barreto A, Songane FF, Lucas M.Is HIV infection associated with an increased risk for cholera? Findings from a case-control study in Mozambique.International Vaccine Institute, Seoul, Korea. Trop Med Int Health. 2008 Mar 6. [11]
Gutiérrez C, Villa S, Mota FR, Calva JJ. Does an L-glutamine-containing, glucose-free, oral rehydration solution reduce stool output and time to rehydrate in children with acute diarrhoea? A double-blind randomized clinical trial. J Health Popul Nutr. 2007 Sep;25(3):278-84.
