Chemistry/Citable Version
Chemistry is the science of matter at the atomic to molecular scale, dealing primarily with collections of atoms (such as molecules, crystals, and metals). Chemists consider matter as made up of individual molecules, and use the known properties of these molecules to predict the outcome of interactions between different substances in bulk. Such interactions are called chemical reactions. Some chemical interactions can require energy, the substances must be mixed, and heat added as well, for a transformation to occur. Other chemical reactions release energy, and these reactions are spontaneous if the substances are brought together in the proper ratios. depending on their chemical properties. The formation of bonds between molecules transforms one substance into another, such as the synthesis of water (H2O) from two gases, hydrogen (H) and oxygen (O).
Introduction
Chemistry is often called the "central science" because it connects other sciences, such as physics, material science, nanotechnology, biology pharmacy, medicine, bioinformatics and geology.[1] These connections are formed through various sub-disciplines that utilize concepts from multiple scientific disciplines. For example, physical chemistry involves applying the principles of physics to materials at the atomic and subatomic level.
Chemistry pertains to the interactions of matter. These interactions may be between two material substances or between matter and energy, especially in conjunction with the First Law of Thermodynamics. Traditional chemistry involves interactions between substances in chemical reactions, where one or more substances become one or more other substances. Sometimes these reactions are driven by a catalyst, which may be another chemical substance present at the reaction (such as sulfuric acid catalyzing the electrolysis of water) or a non-material phenomenon (such as electromagnetic radiation in photochemical reactions). Traditional chemistry also deals with the analysis of chemicals both in and apart from a reaction, as in spectroscopy.
All ordinary matter consists of atoms or the subatomic components that make up atoms; protons, electrons and neutrons. Atoms may be combined to produce more complex forms of matter such as ions, molecules or crystals. The structure of the world we commonly experience and the properties of the matter we commonly interact with are determined by properties of chemical substances and their interactions. Steel is harder than iron because its atoms are bound together in a more rigid crystalline lattice. Wood burns or undergoes rapid oxidation because it can react spontaneously with oxygen in a chemical reaction above a certain temperature.
Substances tend to be classified in terms of their energy or phase as well as their chemical compositions. The three phases of matter at low energy are Solid, Liquid and Gas. Solids have fixed structures at room temperature which can resist gravity and other weak forces attempting to rearrange them, due to their tight bonds. Liquids have limited bonds, with no structure and flow with gravity. Gases have no bonds and act as free particles. Water (H2O) is a liquid at room temperature because its molecules are bound by intermolecular forces called Hydrogen bonds. Hydrogen sulfide (H2S) on the other hand is a gas at room temperature and pressure, as its molecules are bound by weaker dipole-dipole interactions. The Hydrogen bonds in water have enough energy to keep the water molecules from separating from each other but not from sliding around, making it a liquid at temperatures between 0 °C and 100 °C at sea level. Lowering the temperature or energy further, allows tighter organized bonds to form, creating a solid, and releasing energy. Increasing the energy heat of fusion will melt the ice although the temperature will not change until all the ice is melted. Increasing the temperature of the water will eventually cause boiling (see heat of vaporization) when there is enough energy to break the weak polar bonds at 100 °C, allowing the H2O molecules to disperse enough to be a gas. Note in each case, there is energy required to break the bonds, and more energy to move the molecules away from each other.
Scientists who study chemistry are known as chemists. Most chemists specialize in one or more sub-disciplines. The chemistry taught at the high school or early college level is often called "general chemistry" and is intended to be an introduction to a wide variety of fundamental concepts and to give the student the tools to continue on to more advanced subjects. Many concepts presented at this level are often incomplete and technically inaccurate, yet they are of extraordinary utility. Chemists regularly use these simple, elegant tools and explanations in their work because the best solution possible is often so overwhelmingly difficult and the true solution is usually unobtainable.
The science of chemistry is historically a recent development but has its roots in alchemy which has been practiced for millennia throughout the world. The word chemistry is directly derived from the word alchemy; however, the etymology of alchemy is unclear (see alchemy).
History of chemistry
The roots of chemistry can be traced to the phenomenon of burning. Fire was a mystical force that transformed one substance into another and thus was of primary interest to mankind. It was fire that led to the discovery of iron and glass. After gold was discovered and became a precious metal, many people were interested to find a method that could convert other substances into gold. This led to the protoscience called Alchemy. Alchemists discovered many chemical processes that led to the development of modern chemistry. Chemistry as we know it today was invented by Antoine Lavoisier with his law of Conservation of mass in 1783. The discoveries of the chemical elements has a long history culminating in the creation of the periodic table of the chemical elements by Dmitri Mendeleev. The Nobel Prize in Chemistry created in 1901 gives an excellent overview of chemical discovery in the past 100 years.
The chemical industry represents an important economic activity. The global top 50 chemical producers in 2004 had sales of 587 billion dollars with a profit margin of 8.1% and research and development spending of 2.1% of total chemical sales.[2]
Subdisciplines of chemistry

Chemistry typically is divided into several major sub-disciplines. There are also several main cross-disciplinary and more specialized fields of chemistry.
- Analytical chemistry is the analysis of material samples to gain an understanding of their chemical composition and structure. Analytical chemistry incorporates standardized experimental methods in chemistry. These methods may be used in all subdiciplines of chemistry, excluding purely theoretical chemistry.
- Biochemistry is the study of the chemicals, chemical reactions and chemical interactions that take place in living organisms. Biochemistry and organic chemistry are closely related, as in medicinal chemistry or neurochemistry. Biochemistry is also associated with molecular biology and genetics.
- Inorganic chemistry is the study of the properties and reactions of inorganic compounds. The distinction between organic and inorganic disciplines is not absolute and there is much overlap, most importantly in the sub-discipline of organometallic chemistry.
- Organic chemistry is the study of the structure, properties, composition, mechanisms, and reactions of organic compounds. An organic compound is formally defined as any compound bearing one or more covalent bonds between two or more carbon atoms.
- Physical chemistry is the study of the physical and fundamental basis of chemical systems and processes. In particular, the energetics and dynamics of such systems and processes are of interest to physical chemists. Important areas of study include chemical thermodynamics, chemical kinetics, electrochemistry, statistical mechanics, and spectroscopy. Physical chemistry has large overlap with molecular physics. Physical chemistry involves the use of calculus in deriving equations. It is usually associated with quantum chemistry and theoretical chemistry.
- Theoretical chemistry is the study of chemistry via fundamental theoretical reasoning (usually within mathematics or physics). In particular the application of quantum mechanics to chemistry is called quantum chemistry. Since the end of the Second World War, the development of computers has allowed a systematic development of computational chemistry, which is the art of developing and applying computer programs for solving chemical problems. Theoretical chemistry has large overlap with (theoretical and experimental) condensed matter physics and molecular physics. Essentially from reductionism theoretical chemistry is just physics, just like fundamental biology is just chemistry and physics.
- Nuclear chemistry is the study of how subatomic particles come together and make nuclei. Modern Transmutation is a large component of nuclear chemistry, and the table of nuclides is an important result and tool for this field.
Other fields include Astrochemistry, Atmospheric chemistry, Chemical Engineering, Chemo-informatics, Electrochemistry, Environmental chemistry, Flow chemistry, Geochemistry, Green chemistry, History of chemistry, Materials science, Medicinal chemistry, Molecular Biology, Molecular genetics, Nanotechnology, Organometallic chemistry, Petrochemistry, Pharmacology, Photochemistry, Phytochemistry, Polymer chemistry, Solid-state chemistry, Sonochemistry, Supramolecular chemistry, Surface chemistry, and Thermochemistry.
Fundamental concepts
Nomenclature
Nomenclature refers to the system for naming chemical compounds. There are well-defined systems in place for naming chemical species. Organic compounds are named according to the organic nomenclature system. Inorganic compounds are named according to the inorganic nomenclature system.
Atoms
An atom is a collection of matter consisting of a positively charged core (the atomic nucleus) which contains protons and neutrons, and which maintains a number of electrons to balance the positive charge in the nucleus.
Elements
An element is a class of atoms which have the same number of protons in the nucleus. This number is known as the atomic number of the element. For example, all atoms with 6 protons in their nuclei are atoms of the chemical element carbon, and all atoms with 92 protons in their nuclei are atoms of the element uranium.
The most convenient presentation of the chemical elements is in the periodic table of the chemical elements, which groups elements by atomic number. Due to its ingenious arrangement, groups, or columns, and periods, or rows, of elements in the table either share several chemical properties, or follow a certain trend in characteristics such as atomic radius, electronegativity, electron affinity, and etc. Lists of the elements by name, by symbol, and by atomic number are also available. In addition, several isotopes of an element may exist.
Ions
An ion is a charged species, or an atom or a molecule that has lost or gained one or more electrons. Positively charged cations (e.g. sodium cation Na+) and negatively charged anions (e.g. chloride Cl-) can form neutral salts (e.g. sodium chloride NaCl). Examples of polyatomic ions that do not split up during acid-base reactions are hydroxide (OH-), or phosphate (PO43-).
Compounds
A compound is a substance with a fixed ratio of chemical elements which determines the composition, and a particular organization which determines chemical properties. For example, water is a compound containing hydrogen and oxygen in the ratio of two to one, with the oxygen between the hydrogens, and an angle of 104.5° between them. Compounds are formed and interconverted by chemical reactions.
Molecules
A molecule is the smallest indivisible portion of a pure compound or element that retains a set of unique chemical properties.
Substance
A chemical substance can be an element, compound or a mixture of compounds, elements or compounds and elements. Most of the matter we encounter in our daily life are one or another kind of mixtures, e.g. air, alloys, biomass etc.
Bonding
A chemical bond is the multipole balance between the positive charges in the nuclei and the negative charges oscillating about them. More than simple attraction and repulsion, the energies and distributions characterize the availability of an electron to bond to another atom. These potentials create the interactions which holds together atoms in molecules or crystals. In many simple compounds, Valence Bond Theory, the Valence Shell Electron Pair Repulsion model (VSEPR), and the concept of oxidation number can be used to predict molecular structure and composition. Similarly, theories from classical physics can be used to predict many ionic structures. With more complicated compounds, such as metal complexes, valence bond theory fails and alternative approaches, primarily based on principles of quantum chemistry such as the molecular orbital theory, are necessary.
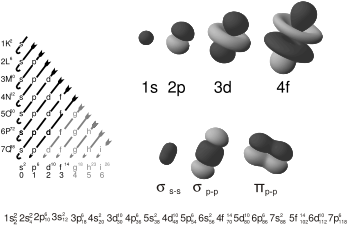
See diagram on electronic orbitals.
States of matter
A phase is a set of states of a chemical system that have similar bulk structural properties, over a range of conditions, such as pressure or temperature. Physical properties, such as density and refractive index tend to fall within values characteristic of the phase. The phase of matter is defined by the phase transition, which is when energy put into or taken out of the system goes into rearranging the structure of the system, instead of changing the bulk conditions.
Sometimes the distinction between phases can be continuous instead of having a discrete boundary, in this case the matter is considered to be in a supercritical state. When three states meet based on the conditions, it is known as a triple point and since this is invariant, it is a convenient way to define a set of conditions.
The most familiar examples of phases are solids, liquids, and gases. Less familiar phases include plasmas, Bose-Einstein condensates and fermionic condensates and the paramagnetic and ferromagnetic phases of magnetic materials. Even the familiar ice has many different phases, depending on the pressure and temperature of the system. While most familiar phases deal with three-dimensional systems, it is also possible to define analogs in two-dimensional systems, which has received attention for its relevance to systems in biology.
Chemical reactions
A Chemical reaction is a process that results in the interconversion of chemical substances. Such reactions can result in molecules attaching to each other to form larger molecules, molecules breaking apart to form two or more smaller molecules, or rearrangement of atoms within or across molecules. Chemical reactions usually involve the making or breaking of chemical bonds. For example, substances that react with oxygen to produce other substances are said to undergo oxidation; similarly a group of substances called acids or alkalis can react with one another to neutralize each other's effect, a phenomenon known as neutralization. Substances can also be dissociated or synthesized from other substances by various different chemical processes.
Quantum chemistry
Quantum chemistry mathematically describes the fundamental behavior of matter at the molecular scale. It is, in principle, possible to describe all chemical systems using this theory. In practice, only the simplest chemical systems may realistically be investigated in purely quantum mechanical terms, and approximations must be made for most practical purposes (e.g., Hartree-Fock, post Hartree-Fock or Density functional theory, see computational chemistry for more details). Hence a detailed understanding of quantum mechanics is not necessary for most chemistry, as the important implications of the theory (principally the orbital approximation) can be understood and applied in simpler terms.
In quantum mechanics (several applications in computational chemistry and quantum chemistry), the Hamiltonian, or the physical state, of a particle can be expressed as the sum of two operators, one corresponding to kinetic energy and the other to potential energy. The Hamiltonian of a particle with no electric charge and no spin is described by the Schrödinger wave equation.
Solutions of the Schrödinger equation for the hydrogen atom gives the form of the wave function for atomic orbitals, and the relative energy of say the 1s,2s,2p and 3s orbitals. The orbital approximation can be used to understand the other atoms e.g. helium, lithium and carbon.
Chemical Laws
The most fundamental concept in chemistry is the law of conservation of mass, which states that there is no detectable change in the quantity of matter during an ordinary chemical reaction. Modern physics shows that it is actually energy that is conserved, and that energy and mass are related; a concept which becomes important in nuclear chemistry. Conservation of energy leads to the important concepts of equilibrium, thermodynamics, and kinetics.
Further laws of chemistry elaborate on the law of conservation of mass. Joseph Proust's law of definite composition says that pure chemicals are composed of elements in a definite formulation; we now know that the structural arrangement of these elements is also important.
Dalton's law of multiple proportions says that these chemicals will present themselves in proportions that are small whole numbers (i.e. 1:2 O:H in water); although in many systems (notably biomacromolecules and minerals) the ratios tend to require large numbers, and are frequently represented as a fraction. Such compounds are known as non-stoichiometric compounds.
Etymology
- Main article: Etymology of alchemy
The word chemistry comes from the earlier study of alchemy, from the old French alkemie; and the Arabic al-kimia: "the art of transformation." An alchemist was called a 'chemist' in popular speech, and later the suffix "-ry" was added to this to describe the art of the chemist as "chemistry".
Alchemy in turn is thought to possibly derive either from Greek word chemeia (χημεία) meaning "cast together", "pour together", "weld", "alloy", etc. (from khumatos, "that which is poured out, an ingot"), or from the Coptic name for Egypt kēme, or alternately, from Persian Kimia meaning "gold".
See also
- Common chemicals - Where to find common chemical components
- List of chemistry topics
- List of chemists
- List of compounds
- List of important publications in chemistry
- Perfection ("Perfection in physics and chemistry")
- Royal Society of Chemistry
- Unsolved problems in chemistry
- Periodic Table of the Elements
External links
- Chemie-Wereld: Startpage (chemistry-world)
- MIT OpenCourseWare | Chemistry
- Chemportal - The navigator for the slovak and czech chemical industry (slovak)
- Wikidchem, The Free Chemistry Archive Wiki
- Chemical Glossary
- http://www.eurochem.eu/ EuroChem (European Portal for Chemistry - Database of Hazard Compounds, European Legislation)
- Chemical Portal
- Chemistry Information Database
- Chemistry Forum
- Chemistry Guide
- Chemistry Conferences
- Chemistry Research
- International Union of Pure and Applied Chemistry
- IUPAC Nomenclature Home Page, see especially the "Gold Book" containing definitions of standard chemical terms
- Experiments videos and photos of the techniques and results
- More experiments - lots of information about the elements too.
- Material safety data sheets for a variety of chemicals
- Material Safety Data Sheets
- Chemical Blogs
For a full list of external links and suppliers see Wikipedia:Chemical sources
References
- ↑ Chemistry - The Central Science. The Chemistry Hall of Fame. York University. Retrieved on 2006-09-12.
- ↑ (July 18, 2005) "Top 50 Chemical Producers". Chemical & Engineering News 83 (29): 20–23.
Further reading
- Chang, Raymond. Chemistry 6th ed. Boston: James M. Smith, 1998. ISBN 0-07-115221-0.
- Pauling, L. The Nature of the chemical bond (Cornell University Press) ISBN 0-8014-0333-2
- Pauling, L., and Wilson, E. B. Introduction to Quantum Mechanics with Applications to Chemistry (Dover Publications) ISBN 0-486-64871-0
- Pauling, L. General Chemistry (Dover Publications) ISBN 0-486-65622-5
- Atkins, P.W. Galileo's Finger (Oxford University Press)
Reading list for university students
- Atkins,P.W. Physical Chemistry (Oxford University Press) ISBN 0-19-879285-9
- Atkins,P.W. et al. Molecular Quantum Mechanics (Oxford University Press)
- McWeeny, R. Coulson's Valence (Oxford Science Publications) ISBN 0-19-855144-4
- Stephenson, G. Mathematical Methods for Science Students (Longman)ISBN 0-582-44416-0
- Smart and Moore Solid State Chemistry: An Introduction (Chapman and Hall) ISBN 0-412-40040-5
- Atkins,P.W., Overton,T., Rourke,J., Weller,M. and Armstrong,F. Shriver and Atkins inorganic chemistry(4th edition) 2006(Oxford University Press) ISBN 0-19-926463-5
- Clayden,J., Greeves,N., Warren,S., Wothers,P. Organic Chemistry 2000 (Oxford University Press) ISBN 0-19-850346-6
- Voet and Voet Biochemistry (Wiley) ISBN 0-471-58651-X