Adenosine: Difference between revisions
imported>David E. Volk (dA is not correct for this molecule, that is 2-deoxyriboadenosnie) |
John Leach (talk | contribs) No edit summary |
||
| (6 intermediate revisions by 2 users not shown) | |||
| Line 10: | Line 10: | ||
|molmass= | |molmass= | ||
|uses= RNA | |uses= RNA | ||
|properties=nucleic acid | |properties=nucleic acid | ||
|hazards= | |hazards= | ||
|iupac= | |iupac= | ||
| Line 20: | Line 20: | ||
== Nomenclature == | == Nomenclature == | ||
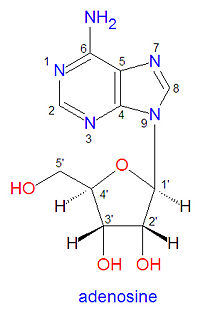
The structure of adenosine contains two distinct parts, the adenine base and the ribose sugar ring. Because of this, RNA and DNA nomenclature use numbers to describe atoms that are on the base ring while using primed numbers to describe atoms on the ribose ring (see figure). In DNA the 2'-carbon of adenosine is bound to two hydrogen atoms rather than one proton and one hydroxyl group. | The structure of adenosine contains two distinct parts, the adenine base and the ribose sugar ring. Because of this, RNA and DNA nomenclature use numbers to describe atoms that are on the base ring while using primed numbers to describe atoms on the ribose ring (see figure). In DNA the 2'-carbon of adenosine is bound to two hydrogen atoms rather than one proton and one hydroxyl group. | ||
==Clinical role== | |||
Adenosine inhibits the effects intracellular [[cyclic AMP]] thus reducing [[sympathetic nervous system|sympathetic]] stimulation.<ref name="isbn0-07-142280-3">{{cite book |author=Keith Parker; Laurence Brunton; Goodman, Louis Sanford; Lazo, John S.; Gilman, Alfred |authorlink= |editor= |others= |title=Goodman & Gilman's the pharmacological basis of therapeutics |edition=11th |language=|chapter=Chapter 34. Antiarrhythmic Drugs |publisher=McGraw-Hill |location=New York |year=2006 |origyear= |pages= |quote= |isbn=0-07-142280-3 |oclc= |doi= |url= |accessdate=}}</ref> | |||
The drug [[dipyridamole]] potentiates adenosine. | |||
The methylxanthines [[caffeine]] and [[theophylline]] block adenosine receptors leading to increased [[sympathetic nervous system|sympathetic]] stimulation.<ref name="isbn0-07-142280-3"/> | |||
==References== | |||
{{reflist}} | |||
[[Category:Reviewed Passed]] | |||
Latest revision as of 05:19, 17 March 2024
|
| |||||||
| adenosine | |||||||
| |||||||
| Uses: | RNA | ||||||
| Properties: | nucleic acid | ||||||
| Hazards: | |||||||
| |||||||
Adenosine, is one of the nucleotides used to build RNA. It is also incorporated into DNA, but in DNA the ribose ring is a 2'-deoxyribose ring. In both DNA and RNA, adenosine is linked to the other nucleotides by phosphodiester bonds at both the 3'- and 5'- positions. In duplex DNA, the adenine base present in adenosine is hydrogen bonded with, that is, it forms a base pair with, a thymidine nucleotide on the opposite DNA strand.
Nomenclature
The structure of adenosine contains two distinct parts, the adenine base and the ribose sugar ring. Because of this, RNA and DNA nomenclature use numbers to describe atoms that are on the base ring while using primed numbers to describe atoms on the ribose ring (see figure). In DNA the 2'-carbon of adenosine is bound to two hydrogen atoms rather than one proton and one hydroxyl group.
Clinical role
Adenosine inhibits the effects intracellular cyclic AMP thus reducing sympathetic stimulation.[1]
The drug dipyridamole potentiates adenosine.
The methylxanthines caffeine and theophylline block adenosine receptors leading to increased sympathetic stimulation.[1]
References
- ↑ 1.0 1.1 Keith Parker; Laurence Brunton; Goodman, Louis Sanford; Lazo, John S.; Gilman, Alfred (2006). “Chapter 34. Antiarrhythmic Drugs”, Goodman & Gilman's the pharmacological basis of therapeutics, 11th. New York: McGraw-Hill. ISBN 0-07-142280-3.
- Pages using ISBN magic links
- CZ Live
- Chemistry Workgroup
- Biology Workgroup
- Health Sciences Workgroup
- Biochemistry Subgroup
- Cardiology Subgroup
- Critical care Subgroup
- Articles written in American English
- All Content
- Chemistry Content
- Biology Content
- Health Sciences Content
- Cardiology tag
- Critical care tag
- Reviewed Passed