Bacillus anthracis: Difference between revisions
imported>Kahtan M Alsaedi |
imported>Kahtan M Alsaedi |
||
| Line 67: | Line 67: | ||
====Fighting Anthrax with Flies==== | ====Fighting Anthrax with Flies==== | ||
[[Image:Anthraxtoxins diagram en.png|thumb|300px|The interactions between the three proteins (PA, EF and LF).]] | [[Image:Anthraxtoxins diagram en.png|thumb|300px|The interactions between the three proteins (PA, EF and LF).]] | ||
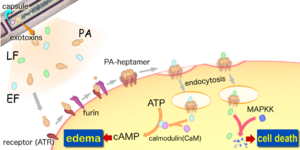
The toxcitiy of Anthrax is due to the secretion of three polypeptides: the Protective Antigen(PA), Lethal Factor(LF), and Edema Factor(EF). The PA is responsible for binding to the surface of target cells. This binding facilitates the relase of LF a metalloprotease that is shown to cleave six of seven Mitogen-Activated Protein Kinase Kinases (MAPKK's). The binding also facilitate the relase of EF a acalmodulin-dependent adenylate cyclase that convert ATP to cAMP. So inorder for the toxcity of anthrax to be felt by the organism, binding between receptors and PA must occur. Lack of binding, Anthrax effect won't be felt, and an organism will survive. Its this quality that Drosophila posses which allows them to survive. The lack of binding is due to the absence of genes that code for receptors in the genome. Regradless of this, Drosophila can be still used to study the effect of anthrax through direct expression of LF and EF. Directed expression allows for greater experimental control, flexibility, and specificity in manipulation of toxins actions invivo. The knowledge of LF and EF function in Drosophila, allows for the testing of known inhibitors and discovering of new drugs against anthrax toxin. | The toxcitiy of Anthrax is due to the secretion of three polypeptides: the Protective Antigen(PA), Lethal Factor(LF), and Edema Factor(EF). The PA is responsible for binding to the surface of target cells. This binding facilitates the relase of LF a metalloprotease that is shown to cleave six of seven [[Mitogen-Activated Protein Kinase Kinases (MAPKK's).]] The binding also facilitate the relase of EF a acalmodulin-dependent adenylate cyclase that convert [[ATP]] to cAMP. So inorder for the toxcity of anthrax to be felt by the organism, binding between receptors and PA must occur. Lack of binding, Anthrax effect won't be felt, and an organism will survive. Its this quality that Drosophila posses which allows them to survive. The lack of binding is due to the absence of genes that code for receptors in the genome. Regradless of this, Drosophila can be still used to study the effect of anthrax through direct expression of LF and EF. Directed expression allows for greater experimental control, flexibility, and specificity in manipulation of toxins actions invivo. The knowledge of LF and EF function in Drosophila, allows for the testing of known inhibitors and discovering of new drugs against anthrax toxin. | ||
====Anthrax, but not ''Bacillus anthracis''?==== | ====Anthrax, but not ''Bacillus anthracis''?==== | ||
Revision as of 09:40, 5 April 2008
Articles that lack this notice, including many Eduzendium ones, welcome your collaboration! |
| Bacillus anthracis | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | ||||||||||||||
| Scientific classification | ||||||||||||||
| ||||||||||||||
| Binomial name | ||||||||||||||
| Bacillus anthracis |
Description and significance
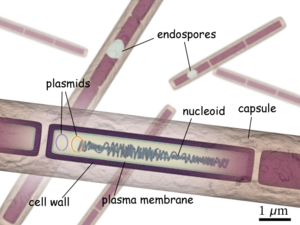
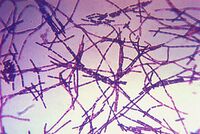
Believed to be responsible for causing anthrax, Bacillus anthracis is a large, rod shaped bacterium. Under ideal conditions, B. anthracis organisms are surrounded by a gel-like covering called capsule. It's this coating that protects it from the immune systems of a host animal, and makes it virulent. Unlike other bacteria, B. anthracis's capsule is made up of poly-D-glutamic acid. Anthrax organisms usually range in size from about 1 to 1.5 microns in width and about 3 to 10 microns in length.
Life cycle of Bacillus anthracis
Life cycle begins with the exposure of the bacterium to harsh conditions such as the death and decomposition of a host which results in the formation of spores. The process of spore formation is dependent on oxygen and thus does not occur in intact corpse. Once spores are formed they are highly resistant to drought, heat, cold, disinfectant, and other unfavorable surroundings. The high resistivity of spores is due to hardy, thick-walled, oval bodies that have an average diameter of about 1-3 microns. Its these characteristics that allow spores to lie dormant in natural environments for hundreds of years. The life cycle continues once the spores are taken up by an animal which then allows them to transform back into a rod-shaped bacteria. The bacteria then begins to multiply and spread through the creature's body. Overtime huge population of germs develop and produce toxin, thus resulting in the host's death, and the reformation of the bacteria into spores.
Types of Anthrax
There are four types of anthrax that are seen in mammals: peracute anthrax, acute anthrax, subacute anthrax, and chronic anthrax. The four types differ in the time it takes from the onset of symptoms to killing the host. With peracute anthrax being the deadliest it kills it's host in a few hours, while Acute anthrax normally takes one to two days from the onset of symptoms to killing it's host. Subacute anthrax lasts from three to five days, while Chronic anthrax usually last more than five days. It has been shown that some animals can recover from the Chronic form of the disease.
Interesting facts about Bacillus anthracis
It was one of the organisms used for the development of the "Koch's postulates". It's importance stems from being able to be isolated in pure culture.
It was the first bacterium to be used for the preparation of a vaccine based on attenuated bacteria by Louis Pasteur in 1881.
At one point scientists distinguished B. anthracis from B. cereus and B. thuringiensis by the presence of pXO1 and pXO2 plasmids. Recent studies however show the "genetic backbone" of both plasmids are not restricted to B. anthracis but can be found in related isolates of B. cereus and B. thuringiensis. These new finding thus raises another dilemma of whether anthrax is caused by B. anthracis or it's a combination of more than one bacteria.
Genome structure
Consisting of a single chromosome, Bacillus anthracis genome is circular and contains approximatley five million base pairs. The virulent factors that are associated with Bacillus anthracis are found on two plasmids pXO1 and pXO2. The plasmids are circular, double stranded DNA molecule, and extrachromosomal. The toxin itself is composed of three plasmid encoded protein. Two of the three proteins are directly toxic, and they are Lethal Factor(LF), and Edema Factor(EF). Increase of LF and EF cause the destruction of white blood cells and the increase in levels of cAMP respectively. Increase in cAMP causes massive edema, thereby imparing energy and water balance. The presence of EF reduces immunity and thus the host becomes susceptible to infection. The third protein is the Protective Antigen(PA) by itself is not toxic. If the PA is inactivated the LF and EF will be harmless. The pXO2 plasmid is resposnible for the production of a capsule.
Cell structure and metabolism
In it's vegatitive state B. anthracies is a gram positive, therefore it contain extensive peptidoglycan layer, lipoteichoic acid, and crystalline cell surface protein (S-layers). What separates B. anthracies from other gram positive bacteria is that it does not conatin teichoic acid and the S-Layers are not glycosylated.
Composed of three polysaccharides: Galactose, N-acetylmannose and N-acetylglucosamine, the cell wall's main function is to anchor the S-layers.
During vegatitive state deprivation of certain nutrient result in endospore formation, which must occur in the presence of oxygen.
When in an aqueous environment, spores germinate and grow.
Has antibacterial killing mechanisms such as hydrogen peroxide formation, defensin synthesis, superoxide dismutase(SOD).
Ecology
Nearly all warm-blooded animals are vulnerable to anthrax. Some of anthrax victims are herbivores especially grazing animals such as cattle and sheep. Anthrax is also seen in demosticated animals such as horses, mules, goats and camels. Anthrax is not limited to just domesticated animals, its also seen in wild animals. On the other hand colded animals are not affected by anthrax at all. The reason for this is because Bacillus anthracis bacteria grows best at temperatures ranging from 77°F to 104°F. Animals whose body temperature does not fall with in the range will be immune to anthrax. A good example of an animal whose body temperature is above the permissible range for Bacillus anthracis and is thus immune to anthrax is a bird.
Pathology
Anthrax can strike at any time, but the most frequent time period when many grazing animals usually get sick is during the dry summer months. Its this time period that available forage decrease. The spores can enter an animal's body when the animal eats a plant along with its root. The coarse vegetation can cause small cuts and abrasions in the mouth, lungs, and intestine of a grazing animal allowing spores to enter the body.
Meat-eating creatures are also at risk of eating spores. They can contract anthrax from consuming sick prey or infected carcasses. Anthrax spores can also be trasmitted by insect bites, infected water, dust blown off anthrax infected soil, and commercial feed made from infected animal carcasses.
Once infection occurs, the incubation period is usually between 3 and 7 days. The course of illness varies with the form of the disease and the animal infected. Death from anthrax usually results from septicemia or blood poisoning which is caused by high levels of Bacillus anthracius organisms and the toxins they secrete. At the time of death, there are on average 10 million to 100 million Bacillus anthracis organisms per milliliter of blood. The presence of the large amount of toxins results in kidney failure, tissue damage, massive swelling, and shock. The shock results from the decline of body functions due to reduced blood circulation.
In the instance where an animal is able to survive an anthrax infection, it will become immune to the disease and thus can not be reinfected.
Current Research
Fighting Anthrax with Flies
The toxcitiy of Anthrax is due to the secretion of three polypeptides: the Protective Antigen(PA), Lethal Factor(LF), and Edema Factor(EF). The PA is responsible for binding to the surface of target cells. This binding facilitates the relase of LF a metalloprotease that is shown to cleave six of seven Mitogen-Activated Protein Kinase Kinases (MAPKK's). The binding also facilitate the relase of EF a acalmodulin-dependent adenylate cyclase that convert ATP to cAMP. So inorder for the toxcity of anthrax to be felt by the organism, binding between receptors and PA must occur. Lack of binding, Anthrax effect won't be felt, and an organism will survive. Its this quality that Drosophila posses which allows them to survive. The lack of binding is due to the absence of genes that code for receptors in the genome. Regradless of this, Drosophila can be still used to study the effect of anthrax through direct expression of LF and EF. Directed expression allows for greater experimental control, flexibility, and specificity in manipulation of toxins actions invivo. The knowledge of LF and EF function in Drosophila, allows for the testing of known inhibitors and discovering of new drugs against anthrax toxin.
Anthrax, but not Bacillus anthracis?
The question of whether Bacillus anthracis is responsible for anthrax arises from recent studies that suggest the similarity between B. anthracis to B.cereus and B. thuringiensis is higher than perivously known. It used to be that the only distinguishing factor of Bacillus anthracis was the presence of two large virulent plasmids pXO1 and pXO2. Recent studies, however show that the genetic backbone for both the pXO1 and pXO2 plasmids are not restricted to Bacillus anthracis but that its found in Bacillus cereus and Bacillus thuringiensis. Isolates of B. cereus continue to show similarity interms of structure of one of the two plasmids present in B. anthracis. The only difference was that the capsule of B. cereus lacks poly-γ-D-glutamic acid. These two isolates and many other isolates, led to the expansion of the phenotypic and biochemical properties that were used to define Bacillus anthacis. Some of those properties were: capsule production, nonmotility, susceptibility to γ-phage, nonhemolyticitly, and susceptibility to penicillin. Even with this expansion some isolates are still mis-designated because of unusual properties. At present, there does not exist any single characteristics or molecular trait that defines one class over another. It used to be that when an organism appears to have anthax-like symptoms Bacillus anthracis was thought to be responsible. Now-adays a thorough exam must be done to rule out the prsence of any B. cereus or B. Thuringiensis.
Factors Involved in the germination and inactivation of Bacillus anthracis spores in murine primary macrophages.
Since it's implication in inhalational anthrax, macrophages have been either used for defense or an enabler of spore movement. They are used for defense, because they efficently sequester spores in the presence of minimal nutrient killing spores that germinate before outgrowth. Following inhalation, spores end up in diverse locations in the trachea and lungs, but a large number get engulfed by phagocytic cells. It was shown in mice that as macrophages level go down, spores germinate more. Upon entry into the vegtative state the capsule formation occur, and as time passes the once spore now a bacterium becomes infective. The experiment began by preparing a primary macrophages that were derived from the bone marrow cells of the femurs of a female mice. After maturation the macrophages were then infected with spores. At different levels of concentration of FBS (Fetal Bovine Serum) and HS (Horse Serum). It was concluded that the extent of germination of spores inside macrophages was dependent on the medium. Aside from being medium dependent, D-alanine was shown to block germination of 70% of the spores in macrophages.
References
Giagtzoglou, N., Bellen, JH. "Fighting anthrax with Flies". Proceedings of the National Academy of Sciences (PNAS). 2006. Volume 103. no.9. p. 3013-3014 [1]
Okinaka, R., Pearson, T., Keim, P. "Anthrax, but not Bacillus anthracis?". PLoS pathogens. 2006. Volume 2. Issue 11. e122. [2]
Hu, H., Emerson, J., and Aronson, AI. "Factors involved in the germination and inactivation of Bacillus anthacis spores in murine primary macrophages". 2007.Volume 272. P245-250. [3]
All the images were taken from Wikimedia commons [4]