Detergent
A detergent is a type of surfacant commonly used in cleaning products like laundry detergents, shampoos and degreasers. The efficacy of detergents is based on the combination of polar hydrophlic groups on one end of the molecule and a hydrophobic group on the other end. Detergents emulsify chemicals in solvents in which they normally would not be soluble. Thus, laundry soap can emulsify grease into water.
Description
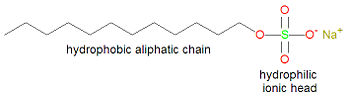
Typically, detergents contain a long alkyl chain of at least twelve carbon atoms and a polar hydrophilic group at the other end (as the sodium salt), other than a carboxylic acid salt. Detergents are often synthetic versions of soap, in which the carboxylic end of soap has been replaced by another hydrophilic group, such as sulfate. This is done to reduce the problem of scum that is associated with using soap in hard water. Whereas soap tends to precipitate in the presence of magnesium or calcium ions, detergents precipitate to a lesser degree and thereby reduce scum.
Sodium lauryl sulfate (also called sodium dodecanyl sulfate), developed from nuts oils, is formed in the reaction of 1-dodecanol with sulfuric acid. It contains both a long, twelve carbon atom hydrophobic alkane section, and a very hydrophilic group, sulfate, on the other end. If the sulfate group were replaced with a carboxylic acid salt, this compound would be called a soap.