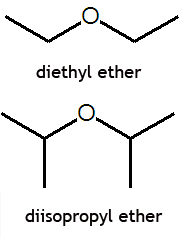
Ether (chemistry)
An ether is a chemical compound in which two hydrocarbons are joined together by an intervening oxygen atom. Ethers, particularly diethyl ether, often called simply ether, and tetrahydrofuran (THF) are common solvents for organic chemistry reactions. Diethyl ether was one of the first anaesthetic agents. Many ethers are extremely flammable, and thus use of diethyl ether in the operating room, in the presence of high oxygen levels, in no longer used.
synthesis of ethers
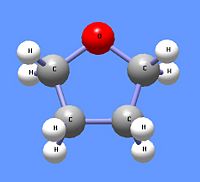
The Williamson synthesis of ethers uses the nucleophilic nature of alkoxide ions to react with primary alkyl halides using an SN2 reaction mechanism. Thus, the reaction of sodium isoproproxide reacts with n-butyl iodide to produce the assymetric ether n-butyl isopropyl ether. Primary alkyl halides are used to minimize the E2 reaction mechanism. The Williamson synthesis can also be used to from cyclic ethers like tetrahydrofuran. To produce cyclic ethers, a primary alkane is used that contains a halide atom at one end and an alcohol on the other end to undergo an intramolecular reaction. The addtion of the strong base sodium hydroxide (NaOH) creates an alkoxide ion from the alcohol. The alkoxide end reacts with the halogenated carbon in an SN2 mechanism, cyclizing the compound while eliminating the halogen atom. Thus, 4-chloro-1-butanol, in the presence of sodium hydroxide, produces tetrahydrofuran.