Halobacterium NRC-1
For the course duration, the article is closed to outside editing. Of course you can always leave comments on the discussion page. The anticipated date of course completion is May 21, 2009. One month after that date at the latest, this notice shall be removed. Besides, many other Citizendium articles welcome your collaboration! |
| Halobacterium sp. NRC-1 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
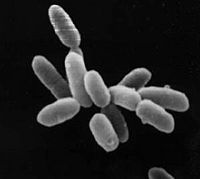 | ||||||||||||||
| Scientific classification | ||||||||||||||
| ||||||||||||||
| Binomial name | ||||||||||||||
| Halobacterium sp. NRC-1 |
Description and significance
Halobacterium sp. NRC-1 is a halophilic archaea which thrives all over the world in high salt environments, including salt production facilities, brine inclusions in salt crystals, natural lakes and ponds, and salt marshes. Halobacterium sp. NRC-1 is motile using both flagella and gas vesicles, and respond to their environment by moving towards chemicals using a process called chemotaxis and toward or away from light using phototaxis using its sensory rhodopsins. They reproduce via binary fission and grow best in a 42 degree Celsius aerobic high salt environment.
Halobacterium sp. NRC-1 is very easy to culture in the lab, and is genetically tractable. It's genome has been completely mapped and whole-genome DNA microarrays are available to investigate gene expression. This makes it an excellent model microorganism for research into the basic cellular process and gene expression as well as for teaching.
Genome structure
The genome of Halobacterium sp. NRC-1 was published in 2000. Since that time, a combination of genetic, transcriptomic, proteomic and bioinformatic approaches have provided insights into both its extremophilic lifestyle as well as fundamental cellular processes common to all life forms.[1]
Halobacterium sp. NRC-1 contains the smallest genome to date among the halophiles. It is 2,571,010 bp in size, and is composed of a large GC-rich chromosome (2,014,239 bp, 68 % G+C), and two smaller extrachromosomal replicons, pNRC100 (191,346 bp) and pNRC200 (365,425 bp), with 58–59 % G+C composition. The two smaller replicons contain 145,428 bp of identical DNA and 33–39 kb inverted repeats catalyzing inversion isomers, and the majority of the 91 IS elements, representing 12 families, found in the genome. As a result of the large number of repeated sequences, genome assembly required extensive genomic mapping and an ordered clone library of pNRC100. Of the 2,630 likely protein-coding genes in the genome, 2,532 are unique. Halobacterium predicted proteins were found to be highly acidic [27] and a substantial number had bacterial homologs as their closest relatives, suggesting that they might have been acquired through lateral gene transfer. In addition, 52 RNA genes were also identified; however, the 16S rRNA sequence and other unique characteristics did not allow placement within a validly described Halobacterium species, and this point has been the subject of some controversy. Interestingly, about 40 genes in pNRC100 and pNRC200 code for functions likely to be essential or important for cell viability (e.g. thioredoxin and thioredoxin reductase, a cytochrome oxidase, a DNA polymerase, multiple TATA-binding proteins (TBP) and transcription factor B (TFB) transcription factors, and the only arginyl-tRNA synthetase in the genome). As a result, these replicons were suggested to be essential "minichromosomes" rather than megaplasmids.[1]
Cell structure and metabolism
Halobacterium species are obligately halophilic microorganisms that have adapted to optimal growth under conditions of extremely high salinity—10 times that of sea water. They contain a correspondingly high concentration of salts internally and exhibit a variety of unusual and unique molecular characteristics. [2] Halobacterium NRC-1 is an aerobic chemoorganotroph, growing on the degradation products of less halophilic organisms as the salinity reaches near saturation. In the laboratory, cells are cultured best in a complex medium. A minimal medium described for Halobacterium includes all but 5 of the 20 amino acids for growth. Several amino acids may be used as a source of energy, including arginine and aspartate, which are passed to the citric acid cycle via 2-oxoglutarate and oxaloacetate, respectively. Under aerobic conditions, arginine is presumably converted to glutamate via the arginine deiminase pathway, and this amino acid then enters the cycle via glutamate dehydrogenase. The arginine deiminase pathway is coded by the arcRACB genes, which are found on pNRC200.[2]
Ecology
Pseudomonas putida has an incomparable effect on the environment. They are able to protect plants from pests, promote plant growth, and clean up organic pollutants found in soil and water.
The surface of the root and the soil that surrounds it are loaded with nutrients released by the plant. This environment is optimal for microbial growth. Pseudomonas putida is attracted to this area, and in turn promotes plant growth and even protects the root against pathogens. Two key elements that allow P. putida to attach in the first place is that they are motile and chemotactic towards the root output. After the initial attraction and migration toward the root, the bacteria immediately begins to grow and divide, forming multiple colonies around the root. The maximum population size is directly related to root weight, and once it is reached the number of colonies will stay constant. All of this can happen in less than 48 hours!
Pseudomonas putida play a huge role in bioremediation, or the removal or naturalization of soil or water contaminants. They can degrade toluene, xylene, and benzene, which are all toxic components of gasoline that leak into the soil by accidental spills. Other strains can convert styrene, better known as packing peanuts, which do not degrade naturally, into the biodegradable plastic polyhydroxyalkanoate (PHA). Methods used to get rid of styrene include incinerating it, spreading it on land, and injecting it underground, all of which release the toxins into the environment. Styrene can cause muscle weakness, lung irritation, and may even effect the brain and nervous system. Due to the fact that P. putida can use styrene as its only source of carbon and energy, it can completely remove this toxic chemical. P. putida can also turn Atrizine, an herbicide that is toxic to wildlife, into carbon dioxide and water.
Application to Biotechnology
Current Research
"Survival and growth of Halobacterium sp. NRC-1 following incubation at -15°C, freezing or freezedrying, and the protective effect of cations"
References
- ↑ 1.0 1.1 DasSarma S et al. (2006), "Post-genomics of the model haloarchaeon Halobacterium sp. NRC-1", Saline Systems 2 (3), DOI:10.1186/1746-1448-2-3
- ↑ 2.0 2.1 Ng, Wailap Victor, et al. (2000-10-24). "Genome sequence of Halobacterium species NRC-1". Proceedings of the National Academy of Sciences of the United States of America 97 (22): 12176-12181. DOI:- 97 VL - 97. Retrieved on 2009-04-18. - 97 Research Blogging.