Potassium in nutrition and human health
To maintain health, the diet of humans must contain potassium, in its ionic form (K+), in double-to-triple-digit millimolar amounts per day. In 2004-2006, the Institute of Medicine of the National Academies of Science[1] and its Food and Nutrition Board[2] recommended that adult humans consume 4700 milligrams (mg) of potassium per day, which, calculated from the atomic mass of potassium (39.1 mg per mmol), amounts to 120 millimoles (mmol) potassium per day: 4700 mg/39.1 mg/mmol =120 mmol.
Subsequent sections will discuss potassium intake requirements for children and special groups.
Humans must regularly consume potassium because the body does not store it (as it does iron and fat, say), while the kidney continues to excrete it in the urine even when potassium intake ceases. Potassium-rich foods include leafy green vegetables, vine fruits (e.g., squash, tomatoes, cucumbers, etc.), root vegetables, and tree fruits (see below).
Potassium ranks as the most abundant cation (positive ion) inside animal cells (intracellular), and as such contributes critically in numerous important ways to the optimal functioning of cells and therefore to optimal functioning of the organ systems and individuals they compose. Among other metabolic functions, potassium plays a role in the synthesis of proteins and in the biochemical transformations required for carbohydrate metabolism.
The ratio of the concentrations of potassium in intracellular fluid (ICF) to that in the cells' surrounding extracellular fluid (ECF) has important effects on the rate of transmission of electrical activity (pulses) along nerve fibers and skeletal muscle cells, and affects the degree of contraction of arteries and arterioles (vascular tone). Inasmuch as extracellular potassium varies in the 3-6 mmol/L range, while intracellular potassium concentrations average about 145 mmol/L, small changes in extracellular potassium concentration have a greater effect on the ICF-to-ECF potassium concentration ratio than small changes in intracellular potassium concentration. Subsequent sections discuss the implication of changes in the ICF-to-ECF potassium concentration ratio in human physiology.
In healthy persons, the amount of potassium consumed equals the amount excreted by the kidney and gastrointestinal tract predominantly.
Disturbances relating to body potassium deficiency may result from:
- inadequate consumption of potassium-containing foods;
- inappropriate excretion of potassium in urine;
- inappropriate excretion of potassium in feces.
Disturbances relating to body potassium excess may result from:
- drugs and kidney diseases that impair the kidney’s ability to excrete potassium in urine;
- deficiency of hormones that act to promote kidney and gastrointestinal excretion of potassium.
Subsequent sections will elaborate on the above introductory concepts.
Requirements for Potassium Consumption by Humans
The Institute of Medicine of the National Academies of Science[1] and its Food and Nutrition Board[2] recommends as Average Intake (AI) of potassium, in mmol/day, as 77 and 97 for children ages 1-3 and 4-8 years, respectively, and as 115 and 120 for children 9-13 and 14-18 years, respectively. For adult men and women, ages 19 to >70 years, they recommend an AI of potassium as 120 mmol/day, and the same amount for pregnant women as young as 14 years, increasing to 130 mmol/day for lactating women.
[...more in progress...]
Potassium Content of Foods
Understanding the biological effects of dietary potassium ions (cations) requires an understanding of the nature of the negatively charged ions (anions) that accompany potassium in foods, balancing potassium’s positive charge and maintaining electroneutrality. In natural diets not subjected to commercial processing that includes addition of potassium salts—typically potassium chloride—a variety of organic anions (e.g., citrate, fumarate) accompany the potassium ions in foods in amounts sufficient to nearly balance the positive charge of the potassium ion (i.e., in near chemical equivalent amounts). Following their absorption by the gastrointestinal tract, a large fraction of those organic anions the body converts to bicarbonate (an acid-neutralizing substance, or base) as an end-product of metabolism. Thus, differing amounts of potassium in the diet exert effects associated and often interacting with the effects of the differing amounts of acid-neutralizing base, bicarbonate, generated by the body. Physiologists often cannot dissect out the specific effects of potassium and bicarbonate when, for example, one increases their dietary intake of potassium-rich foods.
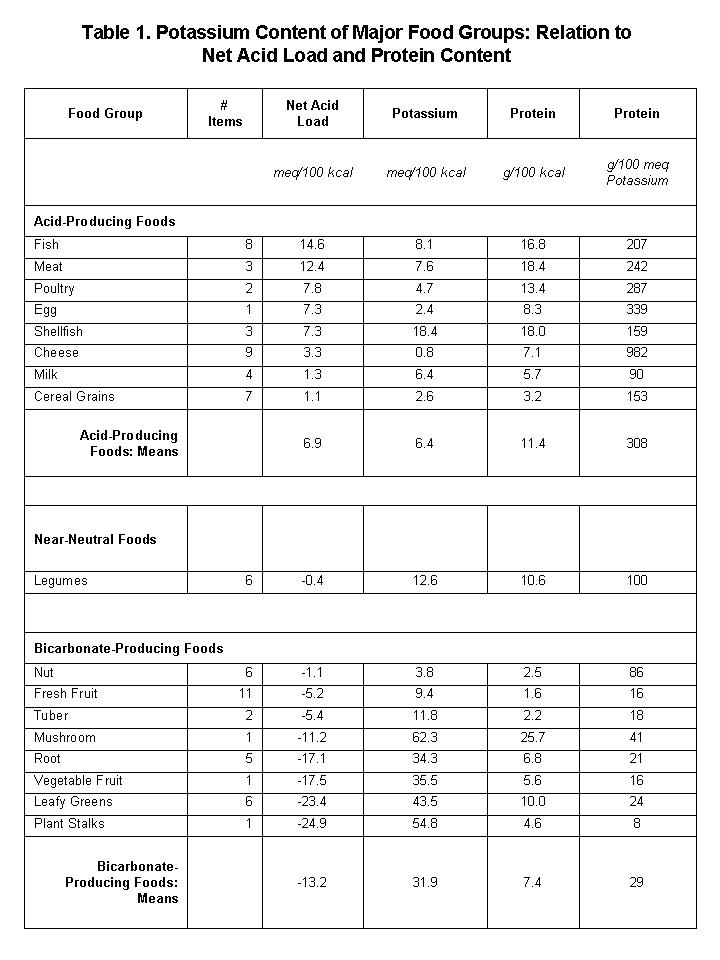
Table 1 shows the potassium content of the major food groups, indicating the relation of potassium content to the net acid (or bicarbonate) load supplied to the body by each food group (see comments following table).
Positive (+) and negative (–) values of net acid load represent acid-producing and bicarbonate-producing equivalents, respectively, in the units specified. Values of net acid load calculated first for individual food items then averaged per food group, as per compositional values in [3]. Acid load calculations as described in:[4] Note that net acid-producing foods tend to have much higher ratios of protein-to-potassium than do net bicarbonate-producing foods (net acid load vs. Protein/Potassium, r=0.48, p=0.05). Note the relatively low values of protein and potassium in the cereal grain group, of which whole grains comprised six of the seven items in the group.
Table 1 reveals a number of important aspects of food potassium:
- Per unit energy content (kilocalories, abbrev kcal), plant foods...
References
- ↑ 1.0 1.1 Otten JJ, Hellwig JP, Meyers LD (editors) (2006) Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. National Academies Press. Pages 370-379. ISBN 0-309-65646-X
- ↑ 2.0 2.1 Panel on Dietary Reference Intakes for Electrolytes and Water. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Food and Nutrition Board. Institute of Medicine of The National Academies (2004) Dietary Reference Intakes For Water, Potassium, Sodium, Chloride, and Sulfate “Potassium” pp. 186-268. The National Academies Press, Washington, D.C.
- ↑ Souci SW, Fachmann W, Kraut H. (2000) Food Composition and Nutrition Tables. Stuttgart, Germany: Medpharm GmbH Scientific Publishers
- ↑ Sebastian et al. American Journal Clinical Nutrition 76:1308-16