Adrenergic beta-agonist
Adrenergic beta-agonists (beta-agonists) are "drugs that selectively bind to and activate beta-adrenergic receptors."[1]
They are divided in several ways: short-acting (SABA) and Long acting adrenergic beta-agonists (LABA).
Medical uses
Asthma
- See Asthma
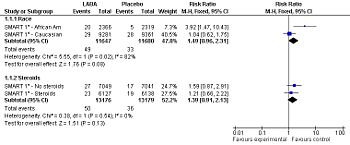
Forest plot: Effect of long-acting adrenergic beta-agonists (LABAs) on the primary outcome of the SMART study[2] (serious adverse event - life threatening adverse events), controlling for race and use of concommitent steroids.
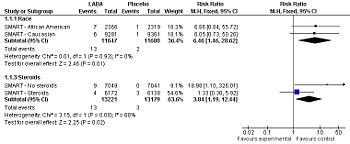
Forest plot: Effect of long-acting adrenergic beta-agonists (LABAs) on the secondary outcome of the SMART study[2] (asthma-related mortality), controlling for race and use of concommitent steroids.
Among patients with asthma, LABAs tend to increase serious adverse effects and mortality. This harm is reduced, but not definitely eliminated, when LABAs are combined with corticosteroids.[3]
Chronic obstructive pulmonary disease
Among patients with COPD, reduction in mortality may not occur unless LABAs are combined with corticosteroids.[4]
Drug toxicity
Long acting adrenergic beta-agonists (LABA) associated with may adverse outcomes among patients with asthma when used without corticosteroids and maybe among African American patients.[5] They might or might not[6] be safe in asthma as long as corticosteroids are used. According to a meta-analyses by the Cochrane Collaboration, when used with corticosteroids the relative risk for asthma-related death is increased at 1.34 although this increase was not statistically significant with a confidence interval of 0.30 to 5.97.[7][3] There are two major randomized controlled trials of LABAs. The SNS study compared salmeterol to salbutamol[8] while the SMART study compared salmeterol to placebo.[2]
The varying response to beta adrenergic agonists in African Americans may be due to a polymorphism in African-American patients of the G protein–coupled cell surface receptor kinase (GRK5)(OMIM) that confers a natural "genetic beta-blockade".[9]
Ozone depletion
In order to comply with ozone protection, these medicines must not use chlorofluorocarbons (CFC) for a chlorofluorocarbon by January 1, 2009.[10][11] Inhalers using hydrofluoroalkane (HFA) propellants are CFC-free. The HFA based inhalers are brand name and more expensive. They are Ventolin by GlaxoSmithKline, ProAir by Ivax, Proventil by Schering-Plough and Xopenex by Sepracor. The first three use albuterol whereas Xopenex uses levalbuterol. Financial assistance is available for patients available through the Partnership for Prescription Assistance (1-888-477-2669).[11] As of November, 2008, the prices costs in American dollars were Ventolin 36.18, ProAir 33.92, Proventil 41.34 and Xopenex 52.50.[12]
References
- ↑ Anonymous (2024), Adrenergic beta-agonist (English). Medical Subject Headings. U.S. National Library of Medicine.
- ↑ Jump up to: 2.0 2.1 2.2 Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM (2006). "The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol". Chest 129: 15–26. DOI:10.1378/chest.129.1.15. PMID 16424409. Research Blogging.
- ↑ Jump up to: 3.0 3.1 Cates CJ, Cates MJ (2008). "Regular treatment with salmeterol for chronic asthma: serious adverse events". Cochrane Database Syst Rev (3): CD006363. DOI:10.1002/14651858.CD006363.pub2. PMID 18646149. Research Blogging.
- ↑ Rodrigo GJ, Nannini LJ, Rodríguez-Roisin R (May 2008). "Safety of Long-Acting {beta}-Agonists in Stable COPD: A Systematic Review". Chest 133 (5): 1079–87. DOI:10.1378/chest.07-1167. PMID 18460518. Research Blogging.
- ↑ Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE (2006). "Meta-analysis: effect of long-acting beta-agonists on severe asthma exacerbations and asthma-related deaths". Ann. Intern. Med. 144 (12): 904-12. PMID 16754916. [e]
- ↑ Salpeter SR, Wall AJ, Buckley NS (2010). "Long-acting beta-agonists with and without inhaled corticosteroids and catastrophic asthma events.". Am J Med 123 (4): 322-8.e2. DOI:10.1016/j.amjmed.2009.07.035. PMID 20176343. Research Blogging.
- ↑ Walters EH, Gibson PG, Lasserson TJ, Walters JA (2007). "Long-acting beta2-agonists for chronic asthma in adults and children where background therapy contains varied or no inhaled corticosteroid". Cochrane Database Syst Rev (1): CD001385. DOI:10.1002/14651858.CD001385.pub2. PMID 17253458. Research Blogging.
- ↑ Castle W, Fuller R, Hall J, Palmer J (April 1993). "Serevent nationwide surveillance study: comparison of salmeterol with salbutamol in asthmatic patients who require regular bronchodilator treatment". BMJ 306 (6884): 1034–7. PMID 8098238. PMC 1676982. [e]
- ↑ Wang WC, Mihlbachler KA, Bleecker ER, Weiss ST, Liggett SB (August 2008). "A polymorphism of G-protein coupled receptor kinase5 alters agonist-promoted desensitization of beta2-adrenergic receptors". Pharmacogenet. Genomics 18 (8): 729–32. DOI:10.1097/FPC.0b013e32830967e9. PMID 18622265. Research Blogging.
- ↑ Anonymous (2008). Information on the Elimination of Chlorofluorocarbon-containing (CFC) Albuterol MDIs and Other Ozone-Depleting Drug Products. Food and Drug Administration.
- ↑ Jump up to: 11.0 11.1 Tarkan L (May 13, 2008). Rough Transition to a New Asthma Inhaler. New York Times. Retrieved on 2008-05-13.
- ↑ [http://www.medicalletter.org/restricted/articles/w1298c.pdf New Propellants for Albuterol Metered-Dose Inhalers] (pdf) 85. Medical Letter (November, 2008).