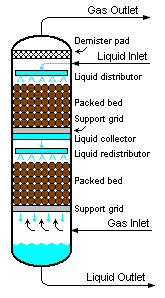
Packed bed
In chemical engineering processes such as distillation and absorption (chemistry), a packed bed is most usually a zone within a vertical pressure vessel that is filled with packing material.[1][2][3][4]
The packing material may be randomly dumped objects or it may be specially designed structured packing material such as the examples shown in Figures 1 and 2.
The randomly dumped packing may be steel, ceramic or plastic objects of various geometric designs. The structured packing may be sheet metal, woven wire gauze or plastic of various designs and stacked in various arrangements.
Applications
In most applications, the purpose of a packed bed is to provide intimate contacting of the upward flowing vapor and the downward flowing liquid in separation processes such as distillation and absorption.
In the packed bed, liquids tend to wet the surface of the packing and the vapors pass across this wetted surface, where mass transfer takes place. Packing material can be used instead of trays or plates to improve separation in distillation columns. Packing offers the advantage of a lower pressure drop across the column when compared to trays or plates, which is especially beneficial when used in vacuum distillation columns.
Differently shaped packing materials have different surface areas and different amounts of void space. Both of these factors affect packing performance. In general, the more surface area for a given volume of packing material, the better is the performance of the packing.
Another factor affecting performance, in addition to packing shape and surface area, is the distribution of vapor and liquid as they that enter the packed bed. The number of theoretical stages required to make a given separation is calculated is a function of the vapor to liquid ratio. If the liquid and vapor are not evenly distributed across the packed bed, the desired separation will not be achieved. The problem is not the packing itself but the mal-distribution of the fluids entering the packed bed. As shown in Figure 3, columns containing packed beds are designed to include liquid distributors so as to distribute the liquid evenly over the cross-sectional area of the packing in order to optimize the efficiency of the mass transfer.[1][2][3] Methods of evaluating the effectiveness of a liquid distributor can be found in the technical literature.[5][6][7]
Packed columns have a continuous vapor-liquid equilibrium curve, unlike conventional tray distillation in which every tray represents a separate point of vapor-liquid equilibrium. However, when designing packed columns it is useful to first determine the number of theoretical equilibrium stages required for the desired separation. Then the packing height needed to constitute a theoretical equilibrium stage, known as the height equivalent to a theoretical plate (HETP), is also determined. The total packing height required is the number theoretical equilibrium stages multiplied by the HETP.[1][2][3][4]
Other applications
Packed beds are also used in certain variants of the distillation and absorption processes referred to as strippers and scrubbers.
Columns used in certain types of chromatography consisting of a tube filled with packing material are called packed columns and their structure has similarities to packed beds.
Trickle filters used for the biochemical oxidation of sewage and industrial wastewaters employ packed beds referred to as fill or media.
Packed bed process design
There are numerous equations and correlations that have been published in the technical literature for predicting the pressure drop of the vapor traveling through a packed bed and for predicting the height equivalent to a theoretical plate (HETP).[2][4] There are also numerous rules of thumb that have been published for use in the process design of packed beds and which are simpler to use and probably as accurate as the equations and correlations.[2] A discussion of all the equations, correlations and rules of thumb would be far beyond the scope of this article.
The major manufacturers of packing material are other sources of reliable process design information.
References
- ↑ 1.0 1.1 1.2 Seader, J.D. and Henley, Ernest J. (2006). Separation Process Principles, 2nd Edition. John Wiley & Sons. ISBN 0-471-46480-5.
- ↑ 2.0 2.1 2.2 2.3 2.4 Kister, Henry Z. (1992). Distillation Design, 1st Edition. McGraw-Hill. ISBN 0-07-034909-6.
- ↑ 3.0 3.1 3.2 King, C.J. (1980). Separation Processes. McGraw Hill. 0-07-034612-7.
- ↑ 4.0 4.1 4.2 Perry, Robert H. and Green, Don W. (2007). Perry's Chemical Engineers' Handbook, 8th Edition. McGraw-Hill. ISBN 0-07-142294-3.
- ↑ Random Packing, Vapor and Liquid Distribution: Liquid and gas distribution in commercial packed towers, Moore, F., Rukovena, F., Chemical Plants & Processing, Edition Europe, August 1987, p. 11-15
- ↑ Structured Packing, Liquid Distribution: A new method to assess liquid distributor quality, Spiegel, L., Chemical Engineering and Processing 45 (2006), p. 1011-1017
- ↑ Packed Tower Distributors: Commercial Scale Experiments That Provide Insight on Packed Tower Distributors, Kunesh, J. G., Lahm, L., Yanagi, T., Ind. Eng. Chem. Res., 1987, vol. 26, p. 1845-1850 Fractionation Research, Inc. (FRI) (Click on "Publications" and then on "K")