Antibiotic
Antibiotics reduce the growth or reproduction of bacteria and are used as medications to treat bacterial infections. They interfere with the life cycle of bacteria in a number of different ways. Some antibiotics, like penicillin, interfere with cell wall synthesis, while others are reverse transcriptase inhibitors that interefere with the production of viral RNA and DNA. Other antibiotics are nucleoside analogs that get incorporated into the viral RNA or DNA and act a chain terminators.
Classes of antibiotics
Penicillins
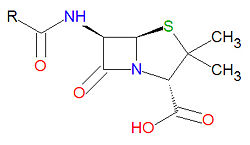
Penicillins have a common beta-lactam base structure, as shown, where R represents different chemical groups. Penicillins work by binding to penicillin-binding proteins irreversibly in a ring-opening reaction and disrupting bacterial cell wall synthesis. Some bacteria are resistant to penicillin because they have acquired the ability to make penicillinases, enzymes which degrade penicillin.
Cephalosporins
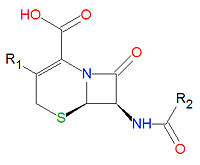
Cephalosporins are a class of antibiotic compounds sharing a common beta-lactam base structure, 7-aminocephalosporanic acid (7-ACA), that was derived from the first cephalosporin discovered, cephalosporin C. Penicillins are very similar, although they contain a five-membered ring in place of the six-membered ring present in the cephalosporin. The activity of cephalosporins, penicillins, and some other antibiotics are due to the presence of a beta-lactam, which binds irreversibly, via acylation, to penicillin-binding proteins, thereby inhibiting the peptidogycan layer of bacterial cell wall synthesis. Cephalosporins are often made semisynthetically. Cephalosporins and the very closely relatedcephamycins are collectively referred to as cephems. In general, second generation and later cephalosporins have a broader spectrum of activity against Gram-negative bacteria.
Because the original cephalosporins used the "ceph" form of the spelling and were often trademarked, the International Nonproprietary Names (INN) suggested by the World Health Organization use the "cef" spelling for the generic drug name of all cephalosporins.
|
Miscellaneous beta-lactams
Several antibiotics contain the beta-lactam ring, but are not considered either penicillins or cephalosporins.
Tetracyclines
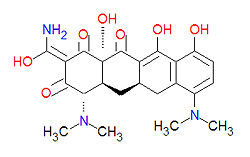
Tetracyclines are antibiotics having a common base structure consisting of four rings conjoined in a linear fashion, with differing chemical groups attached to it, typically on the bottom side or the amino group on the left side in the figure shown. Tetracyclines hinder translation by binding to the 30S ribosomal subunit and preventing the amino-acyl tRNA from binding to the A site of the ribosome, thus disrupting the synthesis of bacterial proteins.
|
Quinolones
The mechanism of action for quinolones is different from that of macrolides, beta-lactams, aminoglycosides, or tetracyclines, so organisisms resistant to those classes of antibiotic drugs may be susceptible to quinolones. In particular, the quinolones interfere with topoisomerase enzymes, including topoisomerase II (DNA gyrase) and topoisomerase IV, which are vital to bacterial DNA replication, transcription, repair and recombination. Because the use of fluoroquinolones may lead to tendinitis or tendon rupture, especially in the Achilles tendon, the FDA requires a "black box" warning for these medications. The cause of the tendon damage is not yet determined.
|
|
Aminoglycosides
All patients taking aminoglycoside antibiotics should be under close observation due to concerns of ototoxicity and nephrotoxicity. These antibiotics have low activity against gram-positive bacteria and are often used in conjuntion with other antibiotics from a different antibiotic class. They function by inhibiting bacterial protein synthesis.
Macrolides and ketolides
Macrolide antibiotics function by binding to the 50S subunit of the bacterial 70S ribosome, thus interferring with the translocation of peptides and the production of bacterial proteins.
- Azithromycin (an azalide, which is a subclass of macrolides)
- Clarithromycin
- Dirithromycin
- Erythromycin
- Roxithromycin
- Telithromycin (a ketolide)
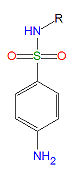
Sulfonamides
Sulfonamides, (R-SO2-NH2) are competitive inhibitors of para-aminobenzoic acid (PABA), the natural substrate for the enzymedihydropteroate synthetase, which is required within the folic acid cycle for the production of folic acid. The sulfonamides are bacteriostatic rather than bacteriocidal. Bacterial resistance to one sulfonamide indicates resistance to all of them.
Glycopeptides
- Bleomycin used as for cancer chemotherapy, not as an antibacterial
- Ramoplanin
- Teicoplanin
- Vancomycin
Oxazolidinones
Other Antibiotic
- Chloramphenicol
- Clindamycin
- Colistin
- Fosfomycin
- Loracarbef
- Metronidazole
- Nalidixic Acid
- Nitrofurantoin
- Polymyxin B Sulfate
- Procaine
- Spectinomycin
- Tinidazole
- Trimethoprim
Antibiotic resistance
A number of organisms have developed resistance to antibiotics. At the microbial level, this may be due either to the antibiotic therapy allowing the survival of naturally resistant organisms of the species, or of the transfer of resistance genetic factors among bacteria.
Assorted human actions cause much of the development of resistance, with reasons ranging to overprescribing antibiotics in situations where they are unlikely to help[1], to patients stopping therapy when they feel better but still have an active bacterial infection, to the use of antibiotics as agricultural growth stimulants.
Regarding overprescribing, one study on respiratory tract infections found "physicians were more likely to prescribe antibiotics to patients who they believed expected them, although they (the physicians) correctly identified only about 1 in 4 of those patients".[2] Multifactorial interventions aimed at both physicians and patients can reduce inappropriate prescribing of antibiotics. [3] Delaying antibiotics for 48 hours while waiting on improvement of respiratory tract infections[4] or cystitis[5] may reduce antibiotic usage; however, this strategy may reduce patient satisfaction.
Normal c-reactive protein levels have been used to help health care providers reduce antibiotic use.[6]
Normal procalcitonin levels have been used to help health care providers reduce antibiotic use.[7][8][9][10]
References
- ↑ Ranji SR, Steinman MA, Shojania KG, Gonzales R (August 2008). "Interventions to reduce unnecessary antibiotic prescribing: a systematic review and quantitative analysis". Med Care 46 (8): 847–62. DOI:10.1097/MLR.0b013e318178eabd. PMID 18665065. Research Blogging.
- ↑ Ong S, Nakase J, Moran GJ, Karras DJ, Kuehnert MJ, Talan DA (2007). "Antibiotic use for emergency department patients with upper respiratory infections: prescribing practices, patient expectations, and patient satisfaction". Annals of emergency medicine 50 (3): 213-20. DOI:10.1016/j.annemergmed.2007.03.026. PMID 17467120. Research Blogging.
- ↑ Metlay JP, Camargo CA, MacKenzie T, et al (2007). "Cluster-randomized trial to improve antibiotic use for adults with acute respiratory infections treated in emergency departments". Annals of emergency medicine 50 (3): 221-30. DOI:10.1016/j.annemergmed.2007.03.022. PMID 17509729. Research Blogging.
- ↑ Spurling G, Del Mar C, Dooley L, Foxlee R (2007). "Delayed antibiotics for respiratory infections". Cochrane database of systematic reviews (Online) (3): CD004417. DOI:10.1002/14651858.CD004417.pub3. PMID 17636757. Research Blogging.
- ↑ Little P, Turner S, Rumsby K, et al (March 2009). "Dipsticks and diagnostic algorithms in urinary tract infection: development and validation, randomised trial, economic analysis, observational cohort and qualitative study". Health Technol Assess 13 (19): iii–iv, ix–xi, 1–73. DOI:10.3310/hta13190. PMID 19364448. Research Blogging.
- ↑ Cals JW, Butler CC, Hopstaken RM, Hood K, Dinant GJ (2009). "Effect of point of care testing for C reactive protein and training in communication skills on antibiotic use in lower respiratory tract infections: cluster randomised trial". BMJ 338: b1374. PMID 19416992. PMC 2677640. [e]
- ↑ Christ-Crain M, Stolz D, Bingisser R, et al. (July 2006). "Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial". Am. J. Respir. Crit. Care Med. 174 (1): 84–93. DOI:10.1164/rccm.200512-1922OC. PMID 16603606. Research Blogging.
- ↑ Stolz D, Christ-Crain M, Bingisser R, et al. (January 2007). "Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy". Chest 131 (1): 9–19. DOI:10.1378/chest.06-1500. PMID 17218551. Research Blogging.
- ↑ Christ-Crain M, Jaccard-Stolz D, Bingisser R, et al. (February 2004). "Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial". Lancet 363 (9409): 600–7. DOI:10.1016/S0140-6736(04)15591-8. PMID 14987884. Research Blogging.
- ↑ Briel M, Schuetz P, Mueller B, et al (October 2008). "Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care". Archives of internal medicine 168 (18): 2000–7; discussion 2007–8. DOI:10.1001/archinte.168.18.2000. PMID 18852401. Research Blogging.
Primary references
- Pages using PMID magic links
- CZ Live
- Health Sciences Workgroup
- Chemistry Workgroup
- Infectious Disease Subgroup
- Pharmacology Subgroup
- Biochemistry Subgroup
- Articles written in American English
- Advanced Articles written in American English
- All Content
- Health Sciences Content
- Chemistry Content
- Infectious Disease tag
- Pharmacology tag